1. 引言
六价铬Cr(VI)是水环境中一种常见的重金属污染物,它容易通过消化道、呼吸道及皮肤接触等途径侵入人体,对人体健康造成危害。随着经济的发展以及消费结构的变化,包括冶金、电气–电镀,涂料和颜料的生产,制革,木材防腐、铬化学品生产和纸浆造纸生产向环境中排放的含铬废水越来越多 [1] 。因此从水环境中去除Cr(VI)是一项重要且有意义的研究,目前对于Cr(VI)的有效去除方法包括化学还原法、生物修复法、石灰固化法及电动修复法等,但是上述方法均存在一定的局限性 [2] 。而吸附法的应用范围较广,是一项比较成熟的水处理技术。
目前已有研究采用林业废弃物、农业废弃物和工业有机废弃物等原料制作相对廉价的吸附剂对Cr(VI)进行吸附研究,均得到较好的效果 [3] 。因此,本文以人工湿地常用水生植物芦苇、香蒲和茭草的秋季枯落物废弃物为原料,将纤维素改性方法应用于芦苇、香蒲和茭草枯落物的改性,以环氧氯丙烷作为醚化剂,在吡啶的催化作用下,与二甲胺发生交联反应,通过接入叔胺基团,改变芦苇、香蒲和茭草枯落物的表面电性。制备高效吸附水中Cr(VI)的改性水生植物枯落物吸附剂。已有研究针对改性秸秆吸附水体磷酸盐以及硝酸盐方面的研究,但是针对改性秸秆对水体重金属尤其是水体六价铬的研究较少 [4] 。
利用这种方式制备的改性水生植物枯落物,以Cr(VI)为目标污染物,通过控制Cr(VI)溶液pH、吸附时间、Cr(VI)初始浓度研究了改性芦苇、香蒲和茭草枯落物的吸附性能,采用动力学方程拟合,吸附等温线拟合的计算初步讨论了吸附机制,为应用改性水生植物枯落物处理含有Cr(VI)的废水提供科学依据。并以期开发一种在工业上可行,且具有成本效益的环境兼容型吸附剂来去除废水中的Cr(VI)。
2. 材料与方法
2.1. 主要试剂与溶液
准确称取0.141 g重铬酸钾固体,用蒸馏水溶解后,移入500 mL容量瓶,配制成0.282 g∙L−1的K2Cr2O7标准溶液,其Cr(VI)浓度为100 mg∙L−1。所用药品均为分析纯。
2.2. 改性水生植物枯落物的制备
原材料改性芦苇、香蒲和茭草枯落物经去离子水洗风干后,用植物粉碎机将原材料粉碎并过100目筛备用。
称取20 g粉碎后保存的改性芦苇、香蒲和茭草枯落物于锥形瓶中,依次加入8 mL N-N-二甲基甲酰胺、12 mL环氧氯丙烷、4 mL二乙烯三胺、8 mL三乙胺,在通风厨中85℃条件下反应4 h,用去离子水反复清洗后抽滤,于空气干燥箱中80℃烘干,即制得即得改性芦苇枯落物ML、改性香蒲枯落物MX、改性茭草枯落物MJ [5] 。
2.3. 吸附实验
2.3.1. 吸附动力学
配制10.0 mg∙L−1的Cr(VI)溶液,准确量取25.0 mL放入分别装有0.1 g改性芦苇枯落物ML、改性香蒲枯落物MX、改性茭草枯落物MJ的离心管中。将离心管置于恒温振荡器中,于25℃,150 r∙min−1条件下避光震荡并开始计时。在10 min、30 min、1 h、2 h、4 h、8 h、16 h、24 h取样,离心,过滤,测定溶液Cr(VI)浓度。
2.3.2. 吸附等温线
配制0.5、1.0、2.0、3.0、5.0、6.0、8.0、10.0 mg∙L−1的Cr(VI)溶液,准确量取25.0 mL不同浓度Cr(VI)溶液至分别装有0.1 g改性芦苇枯落物ML、改性香蒲枯落物MX、改性茭草枯落物MJ的离心管中。将离心管置于恒温振荡器中,分别于15℃、25℃、35℃,150 r∙min−1条件下避光震荡24 h。取出震荡样离心,过滤,测定溶液Cr(VI)浓度。
2.3.3. 溶液pH的影响
配制10.0 mg∙L−1的Cr(VI)溶液,准确量取25.0 mL放入分别装有0.1 g改性芦苇枯落物ML、改性香蒲枯落物MX、改性茭草枯落物MJ的离心管中,溶液pH采用NaOH和HCl调节,使溶液的pH初始值范围在2~10之间。将离心管置于恒温振荡器中,于25℃,150 r∙min−1条件下避光震荡24 h。取出震荡样离心,过滤,测定溶液Cr(VI)浓度。
2.3.4. 分析
用紫外分光光度法测定Cr(VI)浓度。Zeta电位分析仪(ZS90, Malvern Corporation)用于测定AMB的表面电位。实验数据用SPSS 19.0和Excel软件处理,曲线用Origin 8.0软件绘制。
3. 结果与讨论
3.1. 改性水生植物枯落物的基本理化性质
经测定未改性的芦苇枯落物、香蒲枯落物、茭草枯落物均带负电荷,Zeta电位分别为−3.57、−1.26、−2.74 mV。经化学改性的芦苇枯落物、香蒲枯落物、茭草枯落物表面带正电荷,Zeta电位分别为+12.11、+16.42、+10.56 mV。水生植物通过改性接入带正电的叔胺基团,有利于改性芦苇枯落物ML、改性香蒲枯落物MX、改性茭草枯落物MJ对带负电荷的Cr(VI)的吸附。
控制ML、MX、MJ吸附Cr(VI)的吸附能力主要由吸附剂表面的正电荷决定的,通过化学改性,增大了ML、MX、MJ表面正电荷数量,提高了吸附的静电作用力。结果,Cr(VI)被ML、MX、MJ表面通过静电作用吸附。改性后水生植物枯落物秸秆通过秸秆骨架上带正电的叔胺基团与水中阴离子的静电力作用发生吸附,为物理吸附。
3.2. 吸附动力学
吸附动力学的研究可以增强对吸附机理的理解。吸附动力学主要涉及溶液中吸附剂对溶质的吸附速率。为了研究吸附过程的控制机理,本研究采用伪一阶方程和伪二阶方程拟合实验结果。
伪一阶方程:
; (1)
伪二阶方程:
; (2)
qt和qe分别为t时刻和达到吸附平衡后的吸附量,单位为mg∙g−1;t是吸附时间,单位为min;k1和k2是伪一阶方程和伪二阶方程的速率常数,单位分别为min−1和g∙mg−1∙min−1。
如图1所示,ML、MX、MJ对Cr(VI)的吸附效果明显。ML、MX、MJ对Cr(VI)的吸附均呈现出初期快速及中后期缓慢两个阶段,ML、MX、MJ均在初始4 h对Cr(VI)的吸附较快,对Cr(VI)的吸附量迅速上升,并都在8 h吸附基本趋向稳定。
这是因为初期快速阶段,改性水生植物枯落物与Cr(VI)溶液交界面吸附质浓度大,形成了大的吸附动力梯度,Cr(VI)迅速占据改性水生植物枯落物外表面的吸附位点,当Cr(VI)进入改性水生植物枯落物内部孔径后,吸附质穿过外部大孔经过中孔并进一步进入小孔,在此过程中Cr(VI)浓度逐渐减小,吸附动力也随之减小,吸附速率逐渐减慢至吸附平衡。
如表1所示,准二级拟合相关系数R2均比准一级动力学更大,理论平衡吸附量也与实验平衡吸附量接近,由上述分析可知准二级动力学方程能很好的描述改性水生植物枯落物对Cr(VI)的吸附行为,这一结论与其他吸附剂Cr(VI)的动力吸附研究一致。
3.3. 吸附等温线
根据ML、MX、MJ对不同质量浓度Cr(VI)的吸附实验,由平衡质量浓度和吸附量绘制等温吸附曲线,如图2所示,从中可知,无论是何种改性水生植物枯落物,在初始阶段,对Cr(VI)的吸附量随着平衡质量浓度的增加而增大,当平衡质量浓度达到一定值之后,吸附量增加趋势减小,趋于稳定。
吸附等温线有Langmuir方程和Freundlich方程,常用于描述离子在吸附剂上的吸附过程:
Langmuir Equation:
; (3)
Freundlich Equation:
; (4)
其中qe是平衡吸附容量,单位为mg∙g−1;ce是平衡浓度,单位为mg∙L−1;b是Langmuir平衡常数,单位为L∙mg−1;Qm是最大理论吸附容量,单位为mg∙g−1;Kf是Freundlich常数,单位为mg−1/n∙g−1∙L−1/n;1/n是Freundlich指数。
由表2可知,两种方程均能很好的拟合ML、MX、MJ的吸附数据.其中,Langmuir等温线对改性水生植物枯落物的拟合效果更高,表明该吸附过程为单分子吸附。
改性水生植物枯落物的饱和吸附量Qm分别为20.168、19.5957、22.0419 mg∙g−1。
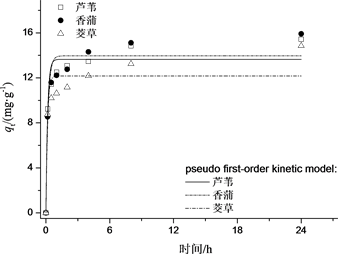
Figure 1. Different kinetic for the adsorption of Cr(VI)
图1. 改性水生植物枯落物对Cr(VI)的吸附动力学拟合曲线

Figure 2. Isotherm fitting of Cr(VI) adsorption
图2. 改性水生植物对Cr(VI)的吸附等温线

Table 1. Kinetic parameters for Cr(VI) adsorption
表1. 标准试验系统结果数据改性水生植物对Cr(VI)的吸附动力学参数

Table 2. Constants and correlation coefficients of Langmuir and Freundlich models for Cr(VI)
表2. 改性水生植物对Cr(VI)的吸附等温线参数
3.4. pH对Cr(VI)吸附的影响以及吸附机制
溶液pH值不仅会影响改性枯落物表面的电荷量、官能团和活性位点,还会影响Cr(VI)的存在形态,因此是吸附过程中一个非常重要的控制因素。
本实验研究了不同初始pH值下ML、MX、MJ对Cr(VI)的吸附效果,结果如图3所示。Cr(VI)的吸附受pH值影响显著,Cr(VI)在ML、MX、MJ上的去除量总体随pH的增加而减小;在pH = 2时,改性水生植物枯落物对Cr(VI)的去除量达到最高;且随着pH的增加,去除量逐渐减小,该结果与现有报道一致 [6] 。
1) 其主要原因在于Cr(VI)在溶液中一般以
、
和
三种阴离子形式存在,这三种离子的含量根据溶液pH的不同而不同,当溶液pH低于6.1时,
是Cr(VI)的主要存在形态 [7] ;随着pH的增大,Cr(VI)的主要存在形式逐渐转变为
。已有研究得出,
由于具有较低的吸附自由能,所以比
更容易被吸附。这是造成在低pH范围内Cr(VI)的吸附量较高的原因之一 [8] 。随着pH上升,由于溶液中OH-的亲和力大于
的亲和力,吸附剂表面的活性点位被
夺取,对Cr(VI)吸附能力下降,甚至不吸附 [9] ;
2) 静电作用也存在于Cr(VI)的吸附过程中。当溶液pH较低时,改性水生植物枯落物表面因发生质子化而带正电荷,有利于对Cr(VI)负离子产生静电吸引 [10] 。随着溶液pH升高,静电吸引力减弱;当溶液pH上升,pH上升使吸附剂表面的官能团发生解离,成为负电性官能团,对同样带负电的Cr(VI)基团产生排斥 [11] 。
3) Cr(VI)的吸附随溶液pH的增大而减弱,这一现象同时也反映了Cr(VI)吸附过程中的氧化还原吸附机理 [12] [13] 。当溶液酸性较强时,Cr(VI)具有很高的正电位和很强的氧化能力,容易与电子供体发生氧化还原反应。所以在酸性条件下,Cr(VI)极易被还原为Cr(III),同时改性水生植物枯落物表面被氧化。Cr(III)通过与改性水生植物枯落物表面含氧酸性官能团之间的交换作用而被吸附 [14] [15] [16] 。
4. 结论
1) 改性水生植物枯落物对Cr(VI)的吸附过程是一个先快速后缓慢的过程,吸附8 h基本达到平衡。

Figure 3. Effects of pH on Cr(VI) adsorption
图3. pH对吸附作用的影响
2) 准二级动力学吸附方程能更好的描述改性水生植物枯落物对Cr(VI)的吸附过程,Langmuir等温线对改性水生植物枯落物的拟合效果更高,该吸附过程为单分子吸附。改性芦苇枯落物、香蒲枯落物、茭草枯落物的饱和吸附量Qm分别为20.168、19.5957、22.0419 mg∙g−1。
3) pH对改性水生植物枯落物对Cr(VI)的吸附影响明显,pH越小越不利于改性水生植物枯落物对Cr(VI)的吸附。
4) 改性水生植物枯落物对Cr(VI)的吸附以离子交换作用、静电作用以及氧化还原作用决定。