1. 引言
目前,商业化的锂离子电池正极材料主要有钴酸锂(LiCoO2)、锰酸锂(LiMn2O4)、磷酸铁锂(LiFePO4)和三元材料等,其中尖晶石LiMn2O4正极材料因其易制备、价格便宜以及对环境友好的优点而被认为是一种很有发展前景的锂离子电池正极材料 [1] [2] 。但是尖晶石LiMn2O4在高温下循环寿命差,容量衰减快,使其商业化受到限制。研究发现,LiMn2O4容量衰减快的原因是充放电过程中锰易溶解在电解液中,导致LiMn2O4材料表面结构发生破坏 [3] [4] 。采用表面包覆能有效阻止LiMn2O4材料与电解液的直接接触,减少锰的溶解。作为包覆材料的化合物一般有:金属氧化物、氟化物、锂离子电池正极材料 [5] [6] 等,其中前人对氧化铝包覆 [7] 的研究相对较多。J. Tu等人 [8] 采用熔盐浸渍法在锰酸锂表面包覆氧化铝,氧化铝的包覆能有效的提高材料循环性能。周宏明等人 [9] 采用化学沉积法在锰酸锂表面包覆氧化铝,氧化铝涂层能有效的降低锰在电解液中的溶解,对材料常温循环性能的提高一定的作用。Feiyan Lai等 [10] 以拟薄水铝石为原料,在锰酸锂表面均匀包覆一层二维纳米状的氧化铝涂层,有效抑制电解液与电极材料的直接接触,提高材料的在常温和高温条件下的电化学性能。氧化铝涂层能有效提高LiMn2O4正极材料的循环性能。本文以九水硝酸铝和锰酸锂为原材料,采用溶胶–凝胶法制备氧化铝包覆锰酸锂材料,通过改变氧化铝的包覆量,研究氧化铝涂层对锰酸锂正极材料结构和电化学性能的影响,从而确定氧化铝的最佳包覆量,拓宽锰酸锂在商业化应用的市场。
2. 实验部分
2.1. LiMn2O4的包覆
按m(Al2O3):m(LiMn2O4)质量比为0.5%,先将Al(NO3)3∙9H2O (西陇化工股份有限公司,AR)溶于一定量的去离子水(市售)中,缓慢加入LiMn2O4 (中信大锰矿业责任有限公司,AR),机械搅拌4 h,再逐滴加入质量浓度为15%的氨水(西陇化工股份有限公司,AR),调节溶液的pH ≈ 9,继续搅拌1 h,抽滤,去离子水洗涤2~3次,80℃烘干,过200目筛网,得到前驱体。将前躯体样品置于管式炉中烧结,在空气气氛中以5℃·min−1的升温速率升温至500℃,保温4 h,自然冷却至室温,即可得到质量比为0.5%的氧化铝包覆锰酸锂样品。采用上述制备前驱体的方法,分别制备氧化铝与锰酸锂质量比为1.0%、1.5%、2.0%和2.5%的氧化铝包覆锰酸锂样品。样品分别标记为0.5%、1.0%、1.5%、2.0%和2.5%。
2.2. 电池的组装
以氮甲基吡咯烷酮(NMP,深圳市新宙邦科技有限公司)为溶剂,将正极活性物质(Al2O3@LiMn2O4)粉末、乙炔黑(广州化学试剂二厂)和PVDF(成都市科隆化工试剂厂,电池级)按质量比为8:1:1混合,打浆,将浆料均匀涂覆在铝箔上,80℃真空干燥4 h。以金属锂片作为对电极和参比电极,LiPF6浓度为1.2 mol∙L-1的电解液(山东海荣电源材料有限公司,电池级),EC与DEC的体积比为1:1。在水和氧含量均在6~10 ppm以下的惰性气氛手套箱中组装电池。
2.3. 性能检测与表征
采用X射线衍射仪(日本理学型号RigakuD)测定样品的XRD 图谱,CuKα射线,管电流100 mA,管电压30 kV,扫描速率为8˚∙min−1,扫描范围10~90˚。用FEI Quanta 200FEG场发散环境扫描电子显微镜观测Al2O3@LiMn2O4复合物的表面形貌,武汉蓝电(LAND 2100)测试电池的循环和倍率性能,交流阻抗及循环伏安曲线在德国ZAHNER IM 6电化学综合测试系统上进行测试。
3. 结果与讨论
3.1. 材料物相结构分析
图1是锰酸锂和不同质量比氧化铝包覆锰酸锂材料的XRD图谱。根据图谱分析可知,锰酸锂样品(a)属于Fm-3d空间群的尖晶石结构,并与锰酸锂(JCPDS No. 35-0782)卡片相对应,也没有检测出其他杂质
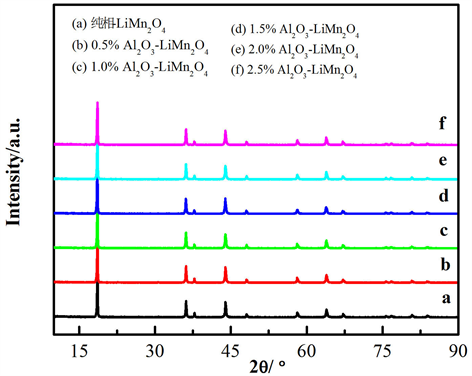
Figure 1. XRD patterns of the pristine LiMn2O4 and different ratio Al2O3-coated LiMn2O4 samples
图1. 锰酸锂和不同质量比氧化铝涂层锰酸锂样品的XRD图
峰。不同质量比氧化铝包覆锰酸锂样品(b-f)与锰酸锂的衍射峰位置及强度相对应,说明氧化铝涂层的包覆并没有改变锰酸锂的晶体结构,可能只是简单的包覆在锰酸锂表面;另一方面,氧化铝的包覆量较少,未达到XRD仪器的最低检出限(大于5%),所以并未检测到氧化铝的峰。
图2为锰酸锂和不同质量比氧化铝包覆锰酸锂材料的SEM图。从图2(a)中可以看到,锰酸锂材料颗粒大小均匀,粒径大小在500~700 nm之间,表面光滑,无杂质。图2(b)图为包覆量0.5%的样品,氧化铝涂层为无序纳米线状,但是并没有将LiMn2O4完全包覆,这对LiMn2O4的保护作用不是很好。图2(c)图为包覆量为1.0 %的样品,氧化铝涂层为无序二维纳米线状,包覆均匀,并相互交错形成均匀的网络结构,既有利于阻止电解液和锰酸锂材料的直接接触,减少锰的溶解;相互交错的网络结构可能为锂离子的传输提供通道,有利于提高电子的转移速率。图2(d)~图2(f)分别是氧化铝包覆量为1.5%~2.5%的样品,氧化铝包覆层在锰酸锂材料表面都出现了不同程度的团聚现象,这很可能会增加电极材料的极化作用,使材料的电化学性能受到影响。

Figure 2. SEM images of LiMn2O4 (a) and different ratio Al2O3-coated LiMn2O4 (b-0.5%; c-1.0%; d-1.5%; e-2.0%; f-2.5%)
图2. 锰酸锂(a)和不同质量比氧化铝涂层锰酸锂材料(b-f)的SEM图
3.2. 电化学性能分析
图3为锰酸锂和不同质量比氧化铝包覆锰酸锂材料的循环寿命图,测试条件为3.0~4.3 V,1 C倍率,25℃(a)和55℃(b)。从图3(a)可以看到,氧化铝包覆的锰酸锂样品循环性能均优于未包覆的锰酸锂。锰酸锂的首次放电比容量较氧化铝包覆锰酸锂的样品高,但是循环200周后其放电比容量仅为72.1 mAh∙g−1,容量保持率为52.78%;当氧化铝包覆量为0.5%时,初始放电容量较低但是200周循环后的容量保持率较高,为77.53%;包覆量为1.0%时,材料具有最好的循环性能,循环200周放电比容量为104.8 mAh∙g−1,容量保持率为92.66%;包覆量为1.5%时,材料的容量保持率为89.87%;包覆量为2%时,容量保持率为87.71%;包覆量为2.5%时,循环200周容量保持率为76.86 %。数据显示,当氧化铝包覆量为1%时,样品具有较好的循环性能。实验结果表明:分散均匀,厚度均一的氧化铝涂层能有效阻止电解液和锰酸锂材料的直接接触,减少锰的溶解;同时,氧化铝涂层在充放电过程中能与Li+反应生成Li-Al-O快离子导体化合物 [11] ,提高材料离子电导率,促进电子的传输,从而达到提高材料循环性能的目的。另外,氧化铝涂层能与电池循环过程中产生的HF反应生成AlF3,AlF3能有效提高材料的离子传输速率,有利于提高材料的电化学性能 [10] [12] 。但是过量的包覆不仅会使氧化铝涂层材料在锰酸锂颗粒表面发生团聚现象,同时增加涂层厚度,导致电极的极化增大,电池内阻增加,导致材料的循环性能下降。图3(b)是6个样品在55℃下循环200周的循环性能图,从图中可以看到,在高温条件下,材料具有较常温低的放电比容量,但是氧化铝包覆的样品均具有较锰酸锂好的高温循环性能。当氧化铝包覆量为1.0%时,材料具有最好的循环性能,200周后容量保持率为96.96%;而锰酸锂循环200周的容量保持率仅为61.29%;其他经过氧化铝包覆的样品均具有较锰酸锂高的容量保持率。实验结果表明:氧化铝涂层在高温条件下具有一定的稳定性,同样能起到保护锰酸锂材料不被电解液腐蚀,减少锰的溶解,提高材料的高温循环性能。
图4为锰酸锂和质量比为1.0%氧化铝包覆锰酸锂材料的循环伏安图,测试条件为25℃,0.2 mV∙s−1。从图中可以看出锰酸锂材料在3.95 V和4.15 V出现了2对不同的氧化/还原峰,这说明锰酸锂材料在充放电过程中,Li+存在两个不同的脱出/嵌入路径,在3.95 V处出现的氧化/还原峰对应着锰酸锂中Li+的脱出/嵌入反应,反应式为:
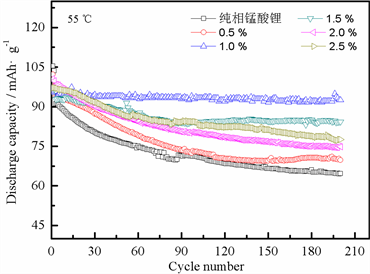
Figure 3. Cycle number of pristine LiMn2O4 and different ratio Al2O3 coated LiMn2O4 at 25˚C and 55˚C
图3. 锰酸锂和不同质量比氧化铝涂层锰酸锂材料循环寿命图
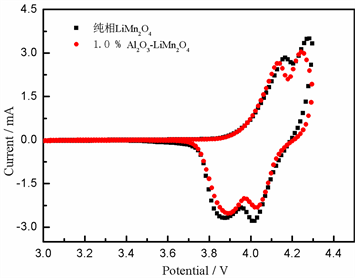
Figure 4. CV and EIS curves of LiMn2O4 and 1.0% Al2O3@LiMn2O4
图4. 锰酸锂和质量比为1.0%氧化铝包覆锰酸锂材料的循环伏安图(a)和交流阻抗图(b)
4.15 V出现的氧化/还原峰对应的是立方相
与四方相Mn2O4的转变,反应式如下
上述两个不同的脱出/嵌入Li+过程是可逆的。1.0%氧化铝包覆锰酸锂材料同样也出现了两个不同的氧化/还原峰,说明氧化铝的包覆并没有改变锰酸锂材料的充放电机制。由图4(a)中可以较直观的看到,氧化铝包覆后的样品具有较锰酸锂材料小的电压差,说明包覆后样品的氧化/还原峰对称性要较锰酸锂的高,材料具有较好的循环性能。图4(b)是锰酸锂和1.0 %氧化铝包覆锰酸锂材料的交流阻抗图,测试条件为25℃,首次充电至4.3 V,微抗电压为5 mV,0.01~105 Hz。从图4(b)中可以看到,交流阻抗曲线分为三个部分:高频区有一个较为完整的半圆弧,主要是固体电解质界面(SEI)膜的形成;中频区也有一个完整的半圆弧,主要是电荷转移过程中所受到的阻碍;低频区为一条具有一定斜率的直线,主要是电极材料中的Li+在固相材料中的扩散速率,对应Warburg阻抗。从图中可以看到,氧化铝包覆量为1%的样品电荷转移速率和Li+的扩散速率要比锰酸锂材料快,电池内阻也有所下降。说明氧化铝在充电过程中能与电池中游离的Li+结合成Li-Al-O快离子导体化合物,同时与电池充电过程中产生的HF反应生成AlF3,有效提高了材料的离子传输速率,降低电池的极化作用,从而提高材料的电化学性能。
4. 结论
采用简单的溶胶–凝胶法成果制备了不同质量百分比的氧化铝包覆锰酸锂正极材料,并研究其结构和表面形貌对锰酸锂正极材料电化学性能的影响。当氧化铝包覆量为1%时,氧化铝涂层为二维纳米絮状,并均匀包覆在锰酸锂颗粒的表面。从XRD和CV曲线图中可以看到:氧化铝的包覆没有改变锰酸锂的晶体结构和充放电机制,适当的氧化铝包覆能有效提高锰酸锂材料的循环性能和高温性能。当氧化铝包覆量为1.0%时,样品表现出较好的循环性能,1 C充放电条件下,200次循环后容量保持率为96.96%。实验结果表明:均匀包覆的氧化铝涂层能有效阻止LiMn2O4和电解液的直接接触,减少锰的溶解;另一方面,均匀的氧化铝涂层有利于在锰酸锂颗粒表面形成均匀的Li-Al-O快离子化合物,提高材料的离子电导率,从而提高电子的转移速率,达到提高锰酸锂材料高低温循环性能及降低电极极化的目的。
基金项目
广西科学研究与技术开发计划(桂科攻1598008-14,桂科AA16380042);广西自然科学基金(2013GXNSFDA019027)。