1. 引言
铅是一种具有神经毒性的重金属,可通过呼吸道、消化道等多种途径进入人体,并在体内积蓄,对人的中枢神经系统、消化系统,特别是肝、肾产生较大危害 [1] [2] 。并且铅有致突变作用,长期食用含铅量高的食品,细胞癌变的危险性增加。因此,亟需研发高效价廉的处理方法治理铅的污染。虽然迄今已研发了化学沉淀法、离子交换法、电化学方法、纳米过滤和吸附法等处理方法 [1] [2] ,但这些方法都存在一些缺点 [3] ,难以在实际重金属废水处理中推广应用。在这些方法中,吸附法因其处理效率高、操作简便、吸附材料来源广、处理成本较低等优点 [4] ,在重金属废水的处理中得到广泛关注和重视。采用吸附法处理含铅废水关键在于吸附剂的性能。在已报道的吸附剂中,天然吸附剂具有价廉、无毒、易得等优点,如天然沸石–高岭土–膨润土等 [5] ,但由于本身结构上的缺陷,对重金属离子的吸附容量较小,选择性低,再生困难,易产生大量含重金属的废弃物而造成二次污染。通过改性虽然有利于提高天然吸附材料对Pb2+的吸附效能,但由于增加了改性步骤,提高了材料制备成本和废水处理成本,如功能化膨润土 [6] 和多孔淀粉 [7] 等。合成吸附材料由于可通过结构设计和可控制备实现吸附功能上的需要,逐步成为研发的重点和热点,如有序介孔炭、超支化聚氨酯树脂、氧化石墨烯、多孔聚合物和介孔二氧化硅及其改性材料等 [8] - [13] 。多孔硅酸钙是由氢氧化钙或钙盐与硅酸盐在一定条件下合成得到的多孔无机硅酸盐材料,由于具有较高白度、高比表面积、高孔隙率和负电荷强度等与众不同的品质特征 [14] ,并且制备原料来源广,工艺简单,成本较低,在水处理中逐渐引起了人们的重视,目前主要用于含磷废水 [15] 、低浓度营养素(N, P) [16] 和焦化废水 [17] 等吸附处理,对重金属废水的处理尚少 [18] [19] 。
本研究以硝酸钙和硅酸钠为原料,十六烷基三甲溴化铵为模板剂,(3-巯基丙基)三甲氧基硅烷为改性剂,采用后接枝法制备巯基化介孔硅酸钙(MCS-SH)。采用XRD、FT-IR、SEM、EDX和BET表面分析等对其结构进行了表征,考察了其对含Pb2+废水的吸附性能,探讨了吸附热力学,取得了较好的结果。本研究为含Pb2+废水的处理提供了一种性能优异的吸附材料。
2. 实验
2.1. 主要试剂和仪器
四水硝酸钙(天津市风船化学试剂科技有限公司)、九水硅酸钠(汕头西陇化工股份有限公司)、十六烷基三甲溴化铵(CTMAB,国药集团化学试剂有限公司)、(3-巯基丙基)三甲氧基硅烷(MPTMS,阿拉丁试剂)、无水乙醇(天津市科密欧化学试剂有限公司)、三乙烯四胺(天津市福晨化学试剂厂)和九水硝酸铅(湘中地质实验研究所)等试剂均为分析纯。实验用水为去离子水。
DZF-6050型真空干燥器(上海精宏实验设备有限公司)、DF-101型磁力搅拌器(巩义市予博仪器设备有限公司)、KYC-111型恒温水浴摇床(上海福玛实验设备有限公司)、TGL
-16G
型高速台式离心机(上海安亭科学仪器厂)。
2.2. 巯基化介孔硅酸钙的制备
准确称取18.5 g九水硅酸钠加入到500 mL蒸馏水中,搅拌1 h使其完全溶解,再按质量比m(CTMAB):m(九水硅酸钠) = 0.05:1缓慢加入
0.925 g
CTMAB,硅酸钠溶液中逐渐出现白色凝胶,继续搅拌1 h。然后按物质的量比n(Ca):n(Si) = 1:1称取
15.4 g
四水硝酸钙,溶解到40 mL蒸馏水中,缓慢滴加到硅酸钠溶液中,溶液中逐步出现白色粘稠物。反应8 h后过滤,用蒸馏水洗涤3~4次,再用无水乙醇在100℃下抽提24 h,然后置于50℃真空干燥箱中干燥24 h,即得介孔硅酸钙(MCS)。
取
2.5 g
MCS加入到40 mL甲苯溶液中,充分搅拌1 h后,用恒压滴液漏斗缓慢滴加5 mL MPTMS,在120℃下反应24 h;然后过滤,依次用乙醇和蒸馏水洗涤3次,置于40℃真空干燥箱中干燥12 h,即得巯基化介孔硅酸钙(MCS-SH)。
2.3. 吸附等温实验
分别准确称量10 mg MCS-SH于不同三角烧瓶中,依次加入50 mL浓度分别为25、50、75、100、125、150 mg∙L−1的Pb2+溶液(未加酸碱调溶液pH值,其pH分别为5.46、5.44、5.41、5.39、5.36、5.32),分别置于293、303、313和323 K下的恒温水浴摇床中,在200 r∙min−1的转速下振荡10 h;然后置于高速离心机上离心,取上层液用原子吸收光谱测定残留的Pb2+浓度。根据吸附前后Pb2+的浓度变化计算Pb2+的吸附量,以吸附平衡时Pb2+的吸附量qe对溶液中Pb2+的平衡浓度ce作图,得到不同温度下的吸附等温曲线。
2.4. pH值对Pb2+吸附的影响
分别准确称量10 mg MCS-SH于不同三角烧瓶中,加入50 mL离子浓度为100 mg∙L−1的Pb2+溶液,以0.1 mol/LHNO3或NaOH调节Pb2+溶液的初始pH值在3.5~7.5。将三角烧瓶置于293 K的恒温水浴摇床中,在200 r∙min−1的转速下振荡10 h;然后离心沉降取上层液,用原子吸收光谱测定残留Pb2+浓度,绘制吸附曲线,考察初始pH值对Pb2+吸附的影响。
2.5. 吸附热力学参数
分别以Langmuir (式1)、Freundlich (式2)和Redlich-Peterson (式3) [20] 吸附模型对2.3吸附实验数据进行拟合,获得相关吸附参数,判断吸附过程符合的模型。
(1)
(2)
(3)
式中:Q为吸附剂表面吸附满单层的最大吸附量,mg∙g−1;b为Langmuir吸附常数,L∙mg−1;KF为Freundlich 常数;n为常数,通常大于1;KR-P、
和
为Redlich-Peterson常数,
介于0~1。
以吸附过程吸附质的分布系数Kd (式4)的对数lnKd对温度的倒数1/T作图,根据式(5)可从直线的斜率和截距分别求出吸附过程的焓变
和熵变
,然后按式(6)可求出吉布斯自由能
,进而判断吸附反应的类型和反应程度 [21] 。
(4)
(5)
(6)
式中:x、y分别为达到吸附平衡时,Pb2+在吸附剂和吸附溶液中的质量,mg;m为吸附剂的质量,g;ν为吸附溶液的体积,mL。因此,x/m和y/ν即分别为吸附平衡时的qe和ce。
2.6. 分析方法
样品物相采用D8 advance型X-射线衍射仪(XRD,德国布鲁克公司)测定;红外光谱采用KBr压片在Spectrum One (B) FTIR红外光谱仪(美国PE公司)测定,波数范围为400~4000 cm−1;硫元素含量采用Vario EL III元素分析仪(德国Elementary Co.)在950℃下测定;氮气吸附–脱附曲线采用BEL SORP II型比表面积及孔容分析仪(日本 BELSOKP公司)在77 K测得,比表面积按照Barrett-Emmett-Teller (BET)方法计算得到;样品形貌采用S-4800场发射扫描电镜(日本日立公司)测定;能谱分析采用Tecnai G
2 F
20型场发射透射电子显微镜(美国FEI公司)测定;Pb2+浓度采用A-Analyst 300型原子吸收光谱仪(美国PE公司)测定。
3. 结果与讨论
3.1. MCS-SH的结构表征
图1~图5分别是MCS-SH的XRD、FT-IR、EDX、SEM和BET谱图,为便于比较将MCS的表征结果一并列入。
从图1可以看出,MCS-SH和未改性的MCS的衍射峰基本上一样,都在2θ = 29.5˚处出现最强衍射峰,在其它地方衍射峰都比较弥散,说明MCS-SH和MCS的结晶度不高,基本上都属于无定形态。与JCPDS卡片比较,MCS-SH和MCS一样都含有CaSi2O5 (PDF# 51-0092),Ca2SiO4·H2O (PDF# 29-0373),和Ca2SiO4 (PDF# 49-1672)物相。以上结果表明巯基改性对硅酸钙的结构基本上没有影响。
从图2中可见,改性前后硅酸钙的FT-IR光谱发生了明显变化:经过MPTMS改性后,在图2(a)中
新出现3个较弱的吸收峰,可分别归属如下 [14] [15] [19] :2935.13 cm−1和1349.93 cm−1分别为-CH2-的不对称伸缩振动峰和弯曲振动峰,在2522.43 cm−1处为S-H键的伸缩振动峰。此外,出现在3400 cm−1左右和1630 cm−1左右分别代表-OH基团的伸缩振动和弯曲振动的吸收峰强度和峰位发生了变化,这是硅烷化后硅酸钙表面的羟基部分被巯基取代所致。以上结果表明,经过改性已将MPTMS中的-SiCH2CH2CH2SH成功修饰到硅酸钙上。其它代表硅酸钙中
(1440 cm−1左右)、骨架中Si-O-Si键的对称伸缩振动(970 cm−1左右)、Si-O四面体的特征吸收(870 cm−1、670 cm−1左右)和Si-O键的伸缩振动(450 cm−1左右)的吸收峰变化很小,说明改性未改变MCS的基本结构。
从图3和表1能谱分析结果可以看出,未经改性的MCS中不存在C和S,而改性后的MCS-SH能谱分析图中出现了C和S的吸收峰,其质量百分比分别为26.042和1.358%,表明改性后硅酸钙中已接入-SiCH2CH2CH2SH基团,印证了红外光谱分析结果。元素分析测定MCS-SH中S的质量分数为1.473%,据此计算得MCS-SH中接入的-SH的量为0.4594 mmol∙g−1。
从图4 MCS-SH(a)和MCS(b)的SEM照片可以看出,未改性的MCS像花瓣密集的花朵,由薄片堆积

Figure 1. XRD patterns of MCS-SH and MCS
图1. MCS-SH和MCS的XRD谱图

Figure 2. FT-IR spectra of MCS-SH (a) and MCS (b)
图2. MCS-SH (a)和MCS (b)的红外光谱图
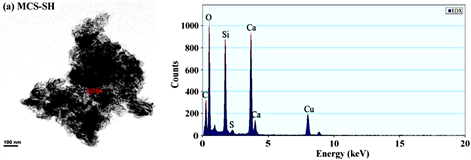
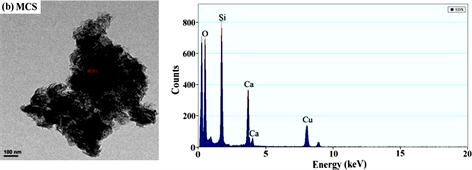
Figure 3. Energy spectrum analysis diagrams of MCS-SH (a) and MCS (b)
图3. MCS-SH (a)和MCS (b)的能谱分析图

Figure 4. SEM images of MCS-SH and MCS
图4. MCS-SH和MCS的SEM照片

Figure 5. Nitrogen adsorption-desorption isotherms (a) and pore size distribution curves (b) of MCS-SH and MCS
图5. MCS-SH和MCS的氮气吸附-脱附曲线(a)和孔径分布图(b)
构成形状各异的孔道,表面凹凸不平;而改性的MCS-SH基本结构与MCS相似,只是孔道中有些小的颗粒填充;二者都是无定形的,与图1的XRD分析结果一致。这些丰富的孔道和凹凸不平的表面为重金属离子的吸附提供了丰富的场所。以上结果也表明,在改性过程中虽然有少量小颗粒填充在孔道中,但MCS的基本结构保持不变,因此,MCS在改性过程中能维持稳定的结构,具有较高的稳定性。
从图5可以看出,MCS-SH和MCS的吸附/脱附曲线属于IUPAC定义的六种吸附曲线中第Ⅴ类吸附曲线,带H3型滞后环。结合图4的SEM照片可知,MCS-SH和MCS的孔道都是由薄片堆积而成的狭缝状孔道。从图5(b)的孔径分布可以看出,MCS-SH和MCS的孔径分布均比较集中,其孔径范围分别为5~49 nm和4~50 nm,为介孔。MCS-SH的比表面积和孔径比MCS分别减少了28.81 m2∙g−1和2 nm (表2)。其主要原因可能是:一方面改性后MCS-SH中接入了-SiCH2CH2CH2SH基团,另一方面是在改性过程中造成了少部分硅酸钙薄片破损而填塞在原硅酸钙的孔道中(图4),使比表面积和孔径减少。
3.2. 温度对吸附性能的影响
图6给出了MCS-SH对Pb2+的等温吸附曲线。从图6可以看出,在相同温度下,当吸附溶液中Pb2+浓度低于100 mg∙L−1 (第4个实验点)时,Pb2+的吸附量均随吸附溶液中Pb2+浓度的增加而快速增加;当浓度达100 mg∙L−1时,吸附量的变化趋于平缓,再增加浓度,吸附量增加不大。MCS-SH对Pb2+的吸附等温线均随着温度升高而上移,表明吸附量随温度升高而增加;但温度由313 K升高323 K时,吸附量增加的幅度明显低于313 K以下增加的幅度。由此可见,升高温度对吸附有利,MCS-SH对Pb2+的吸附为吸热反应。造成在较高温度下(323 K)吸附量增加幅度降低的原因可能是,MCS-SH的吸附不仅是单纯的化学吸附,还有物理吸附;温度升高,物理吸附的Pb2+的量减少,而化学吸附的Pb2+的量增加;在较低温度下,随温度升高由化学吸附引起的吸附量增加占主导,而在较高温度下,由物理吸附产生的吸附量降低变得更明显,从而导致吸附量增加幅度明显降低。
采用Langmuir、Freundlich和Redlich-Peterson模型分别对图6数据点进行拟合,结果如表3所示。从表3可以看出,拟合的相关系数以Redlich-Peterson模型最高,Langmuir模型次之,Freundlich模型最低。因此,Redlich-Peterson模型和Langmuir模型均能对实验数据进行较好地拟合,MCS-SH对Pb2+的吸

Table 1. Energy spectrum analysis results of MCS-SH (a) and MCS (b)
表1. MCS-SH (a)和MCS (b)的能谱分析结果

Table 2. The specific surface area and pore size of MCS-SH and MCS
表2. 样品的比表面积和孔径
附更符合Redlich-Peterson模型。这是因为Redlich-Peterson模型是综合了Langmuir模型和Freundlich模型的成功之处,不受Langmuir模型理想单层假定的约束 [20] ,因此更适于描述不均匀表面的物理吸附和化学吸附。根据Langmuir吸附模型,在293 K下,MCS-SH对Pb2+的最大吸附容量为618.09 mg∙g−1,比未改性的MCS高(544.84 mg∙g−1),远高于文献报道的常见吸附剂(见表4)。
3.3. MCS-SH对Pb2+吸附的热力学特征
图7给出了吸附平衡时Pb2+的分布系数Kd的对数lnKd对(1/T)的关系曲线,其中实线为293 K、303 K、

Figure 6. The adsorption isotherms of MCS-SH for Pb2+
图6. MCS-SH对Pb2+的吸附等温线

Table 3. Nonlinear fitting parameters of MCS-SH for Pb2+
表3. MCS-SH吸附Pb2+的非线性拟合参数

Table 4. The adsorption capacities of adsorbents towards Pb2+ in the literatures
表4. 文献报道的吸附剂对Pb2+的吸附容量
313 K和323 K等4个温度的拟合曲线,虚线为前3个温度的拟合曲线。根据拟合直线的截距和斜率求得吸附热力学参数ΔS、ΔH和ΔG列入表5中。从图3可以看出,MCS-SH对Pb2+吸附在前3个温度下的线性拟合相关系数高于4个温度下拟合曲线的相关系数,表明在温度低于313 K时,对Pb2+的吸附能很好地遵从式(5)所示的关系规律;当温度达323 K时,对Pb2+的吸附明显偏离式(5)所示的关系规律。这是因为MCS-SH对Pb2+的吸附包含化学吸附和物理吸附,当温度较高时(323 K),由物理吸附引起的随温度升高而降低的量比低温下随温度升高而降低的量更多,从而出现明显偏离。
从表5可以看出,MCS-SH对Pb2+吸附过程的ΔH和ΔS均大于0,ΔG均小于0,说明对Pb2+的吸附为吸热熵增的自发过程。这是因为MCS-SH除有较大的比表面积和丰富的孔道外,表面还有丰富的活性基团,如-OH、-O等 [14] ,MCS-SH还修饰上对Pb2+具有更强亲和力的-SH,对Pb2+的吸附以这些活性基团与Pb2+发生配位作用的化学吸附为主,因此需要吸收一定能量克服活化能而表现出吸热现象。由于化学吸附只占吸附的一部分,因此,吸热量不大。导致MCS-SH对Pb2+吸附熵增加的原因可能是Pb2+在水溶液中是溶剂化的,吸附剂在制备过程中总会吸附一定量溶剂分子,在加入到吸附液中时也会很快被溶剂分子所包围,当Pb2+吸附到吸附剂表面时,对Pb2+而言混乱程度减少,但Pb2+的溶剂化层的溶剂分子以及吸附剂表面的溶剂分子会部分释放出来,由于后者大于前者,使整个体系的混乱程度增加。
3.4. pH值得影响
图8给出了MCS-SH用量均为10 mg、吸附时间为10 h时,溶液初始pH值对吸附效果的影响。可以看出,当溶液初始pH值在5.0~7.5时,对Pb2+都有较高的吸附率;当pH值低于5时,吸附率随pH值的升高而迅速增加;当初始pH达到5以上时,吸附率开始趋于稳定,MCS-SH对Pb2+的吸附率达95.46%。在未加酸碱调节溶液pH时,100 mg/L的Pb2+溶液初始pH值为5.39,此时的吸附率为97.96%;继续提高初始pH值,吸附率增加不明显,因此,适宜的pH值范围为5.0~7.5。

Table 5. The thermodynamic parameters of Pb2+ adsorption on MCS-SH
表5. MCS-SH吸附Pb2+的热力学参数

Figure 7. LnKd vs. 1/T for Pb2+ adsorption on MCS-SH
图7. MCS-SH吸附Pb2+的lnKd与1/T关系
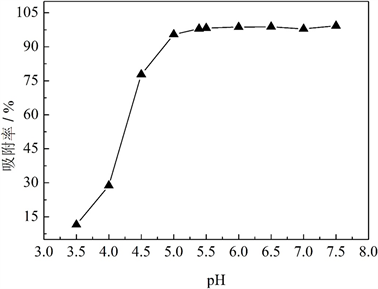
Figure 8. Effects of the initial pH values of Pb2+ ion solution on the removal rates for Pb2+
图8. Pb2+溶液初始pH值对Pb2+吸附效果的影响
4. 结论
1) 以硝酸钙和硅酸钠为原料,十六烷基三甲溴化铵为模板剂,(3-巯基丙基)三甲氧基硅烷为改性剂,采用后接枝法制备巯基化介孔硅酸钙(MCS-SH)。MCS-SH在改性中维持了较稳定的介孔结构,吸附/脱附曲线属于第Ⅴ类吸附曲线,带H3型滞后环,为夹缝孔;比表面积为129.32 m2∙g−1,孔径范围为5~49 nm,比MCS的表面积减少28.81 m2∙g−1,孔径减少2 nm;MCS-SH中接入的-SH量为0.4594 mmol∙g−1。
2) MCS-SH在pH 5.0~7.5时对Pb2+都具有优良的吸附性能;在293 K下,MCS-SH对Pb2+的最大吸附容量为618.09 mg∙g−1,明显高于MCS的最大吸附容(544.84 mg∙g−1),远高于常见文献报道的吸附剂。MCS-SH对Pb2+的吸附符合Langmuir模型,更适合Redlich-Peterson模型,为一个熵增自发进行的过程,吸热反应,存在物理吸附和化学吸附。MCS-SH有望成为一种性能优良的Pb2+吸附材料。
基金项目
感谢国家自然科学基金资助(No. 51378201)和湖南省教育厅科学研究重点项目(No. 16A069)对本工作的资助。