1. 引言
在纳米含能材料研究领域,纳米铝热剂,又称亚稳态分子间复合物(Metastable intermolecular composites,MIC),或超级铝热剂(Super thermites),具有体积能量密度高、燃烧速率快、反应温度高等优良性能,已经引起了国内外研究者的广泛关注 [1] 。
针对MIC材料的制备、性能、机理等方面,国内外学者做了诸多研究。如Sanders [2] 研究了Al/Bi2O3、Al/CuO、Al/MoO3、Al/WO3四种MIC复合物的制备和反应性能。Piekiel等 [3] 探索了纳米铝热剂Al/Bi2O3反应的点火过程,通过“反应烧结”研究,证明了固相反应是纳米铝热反应的主要形式,且是纳米铝热剂Al/Bi2O3点火的原因。Zachariah等 [4] 采用分子动力学模拟(MD)研究了纳米铝热反应预点火之前反应物粒子的熔融结合机理,很好地解释了当反应粒子足够小时反应燃烧时间不再减少的实验现象。Baijot等 [5] 基于局部热力学平衡建立了MIC材料的压力输出模型。Shaw等 [6] 建立了纳米复合物Al/MoO3的高速燃烧波的爆轰波模型和一维C-J爆轰模型,模拟结果表明,多相混合物的平衡声速在纳米复合材料Al/MoO3的快速燃烧中具有关键作用。Martirosyan等 [7] 对MIC材料的输出动力学进行了模拟研究,为了对反应进行简化,假设反应产生了一维爆轰波,并以此形式进行传播,建立了MIC材料反应的流体力学模型,该模型基于气体动力学方程可以对反应过程中的压力演变进行预测。
目前,对于MIC材料的研究仍集中在制备方法、性能改进方面,对于燃烧过程的数值分析还处于起步阶段,国内更是鲜有报道 [8] 。为了探究MIC化学燃烧反应的特性及其影响因素,本文从传热传质和流体力学角度,对以Al/Bi2O3为代表的MIC复合材料点火及燃烧波传播过程进行模型简化,建立相应的数学计算模型,进行数值计算分析。
2. 燃烧特性计算模型
2.1. 物理模型
Al和Bi2O3的化学反应方程式如式(1)所示,其中
表示反应进度。
(1)
数值计算以Al/Bi2O3反应化学方程为基础,采用电点火方式引燃点火药 [9] ,点火药产生热量与燃烧波,作用于Al/Bi2O3反应物外边缘。在反应区内,边缘Al粒子与Bi2O3粒子受热迅速发生化学反应,产生大量热和燃烧波,进而引发复合材料自持性燃烧反应。自持燃烧波的典型燃烧特征物理模型如图1所示 [10] [11] 。热传递方向由反应区到未反应区,温度由反应后温度Tc逐渐变为反应前温度T0,反应进度η由0很快变成1。
基于如上物理过程,引入如下假设,简化计算模型:
1) 模拟以Al/Bi2O3反应化学方程式为基础,忽略中间产物影响;
2) 假设化学反应产生一维均匀燃烧波,并且波阵面厚度远大于反应物粒子的直径;
3) 假设反应物之间均是固相反应,不考虑反应过程中的相变;
4) 假设反应物装药为均匀的球体,反应物孔隙间空气对传热传质的影响忽略不计;
5) 反应过程不受相变、粒子微观结构等因素的干扰。
2.2. 数学模型
在预反应区发生热扩散过程,反应能量方程和热扩散模型状态方程可以表达为:
(2)
其中,
为混合物实际密度,kg/m3;
为混合物比热,J/(kg·K);
为反应温度,K;
为混合物热传导率,W/(m∙K);
为Al/Bi2O3反应单位质量放热焓;
为反应区反应速率,1/s;
表示位置,m;
表示时间,s。
混合物总热传导率
由如下公式进行计算 [12] :
(3)
其中,k1、k2分别为Bi2O3和Al的热传导率,ψ为Al和Bi2O3的体积比。
混合物的实际密度
。θ为混合物的孔隙率,在计算中,孔隙率随配比和粒径的变化忽略不计;ρ0表示混合物的理论密度,由式(4)计算得到。
(4)
其中,M1、M2分别为Al和Bi2O3的相对原子(分子)质量;ρ1、ρ2分别为Al和Bi2O3的密度,kg/m3;y表示Al和Bi2O3的摩尔配比。
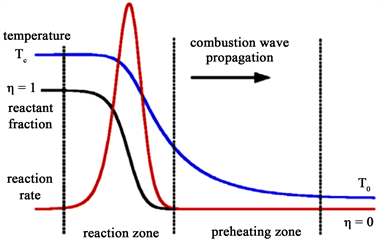
Figure 1. Preheating and reaction zones in nanoscale thermite reaction
图1. 反应区与预反应区示意图 [11]
混合物的热容c由Al和Bi2O3的热容(分别为c1、c2)确定:
(5)
Al/Bi2O3反应单位质量放热焓由式(6)计算得到:
(6)
其中,H0表示理论化学计量比Al/Bi2O3的单位质量放热焓,2.12 kJ/g。
另外,根据阿伦尼乌斯方程,化学反应速率与活化能、温度有如下关系式:
(7)
其中,
为反应物剩余分数,
;
为化学反应活化能,J/mol;A为指前因子,s−1,由化学反应活化能确定 [12] 。
在此模型下,反应燃烧波的燃速由如下公式计算:
(8)
其中,
表示燃烧波速度,m/s;
表示位置函数。
3. 结果与讨论
采用COMSOL Multiphysics软件一维对称物理场固体传热模块和化学反应模块进行数值模拟求解,耦合类型选择内部热源,边界条件为点热源,采用瞬态研究方法可求得反应场温度T、化学反应速率φ、反应程度η,绘制温度、反应速率随时间、空间变化曲线。相关计算参数如表1所示。
3.1. Al/Bi2O3燃烧特性数值计算结果与分析
以理论化学计量比(Al与Bi2O3的摩尔比为2:1)、Al粉粒径为40 nm、Bi2O3粒径为47 nm的Al/Bi2O3复合材料为例,计算反应温度随时间、位置的变化关系,结果如图2所示。

Table 1. Parameters in simulation of combustion characteristics of nanothermite Al/Bi2O3
表1. Al/Bi2O3燃烧特性计算参数值
注:Ea1表示平均粒径为40 nm的理论化学计量比Al/Bi2O3活化能,Ea2、Ea3、Ea4分别表示平均粒径为24 nm、100 nm、2000 nm的理论化学计量比Al/Bi2O3活化能,由Al粒径变化对活化能影响的关系 [14] 确定;θ表示混合物孔隙率;Δt、Δx分别表示瞬态求解过程的时间、空间变化步长

Figure 2. Temperatures as a function of time and location in Al/Bi2O3 reaction
图2. Al/Bi2O3反应区温度随时间、位置变化曲线图
如图2中所示,燃烧波形图与图1燃烧模型示意图一致,说明计算结果与计算模型相吻合,在一定程度上可以模拟实际燃烧过程。整个燃烧过程分为以下三个阶段。
1) 燃烧前升温阶段(£32.653 m/s):此阶段反应区温度较低,未达到点火温度,Al/Bi2O3化学反应速率较低,温度随着热源输入逐渐上升直至点火温度。
2) 燃烧反应阶段(32.654~32.660 m/s):此阶段反应区温度达到了点火温度,Al/Bi2O3发生自持燃烧反应,形成燃烧波阵面,并放出大量热。反应放出的大量热量使反应区温度迅速上升,达到较高的温度输出。反应到达点火温度的时间点定义为点火(延迟)时间,用tini表示。
3) 燃烧后热力学局部热力学平衡阶段(>32.660 m/s):此阶段随着反应进程接近于1,化学反应速率减小,放热减小,达到局部热力学平衡,温度基本不再发生变化。
Al/Bi2O3反应达到局部平衡时的计算温度平均值为4217 K,燃烧波传播计算平均速度为671.4 m/s,这两者的实际测量值分别为3592 K和617 m/s [15] 。计算平衡温度高于实际测量温度值,这是因为实验和数值计算的点火热量输入值大小不同 [16] 。在实际反应过程中,当反应物升温至Al熔点(约933 K)时,Al熔化成液态,即与Bi2O3开始发生反应 [12] 。而在模拟计算过程中,由于通过式(4)化学反应速率的变化来控制燃烧过程的发生,温度高于1200 K时化学反应速率才发生突变跃迁,因此在计算中需要输入更多的热量,加热更长的时间,导致平衡温度高于实际测量值,且延迟时间也大于实际值。对于燃烧波速度,计算值与实验值基本吻合,同时也验证了计算模型的正确性。
Al/Bi2O3化学反应速率随时间、位置的变化关系如图3所示。图中每一条曲线表示该时刻下、不同位置处的化学反应速率。图3中曲线的最高值称为该时刻化学反应速率最大值,即最大反应速率,各曲线最大反应速率的平均值为平均最大反应速率,用φmax表示。
如图3中所示,反应速率波形图与燃烧波位置变化图相对应,当燃烧反应传播到某一位置时,该位置处的反应速率急剧增大,达到最高之后,由于反应进程逐渐增大,反应速率随之减小。不同位置达到化学反应速率最高值的时间不同,但是各位置点的反应速率变化曲线及最大反应速率基本相同。按图1所示的燃烧面模型示意图,反应的反应区与预反应区总宽度约为0.1 cm,远大于反应物粒径40 nm,即符合模型假设条件。
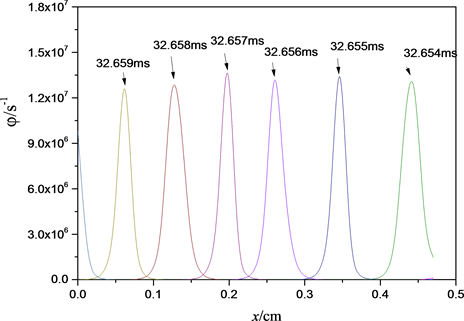
Figure 3. Chemical reaction rates as a function of time and location in Al/Bi2O3 reaction
图3. 反应速率随时间、位置变化曲线图
3.2. Al/Bi2O3燃烧特性影响因素
3.2.1. 配比
当反应物粒径等因素保持不变时,以40 nm Al粉、47 nm Bi2O3的复合材料为例,Al和Bi2O3的摩尔比为1.5、1.8、2、2.5、3、4的Al/Bi2O3反应点火端的温升曲线如图4所示。可以看出,各条曲线升温过程基本相似,均经历了加热升温阶段、发火升温阶段和局部温度平衡阶段。在30 m/s之前的加热升温阶段中,Al粉含量较低的复合物在相同时间的温度更高。这是由于Al粉的传热速率大于Bi2O3粉末的,因此具有高Al/Bi2O3配比的复合物也具有高的传热速率。在相同的加热功率条件下,配比较高的复合物Al粉含量多,热量更快地扩散到复合物中,导致加热点的升温速率较低。
在点火升温阶段,Al和Bi2O3的摩尔比为1.5、1.8、2、2.5、3、4的复合物反应点火时间tini分别为36.175 ms、35.430 ms、32.654 ms、33.653 ms、35.836 ms、39.632 ms,如图5所示。不同配比的复合物装药量相同,反应物配比为理论化学计量比的复合物具有最小的反应点火时间。这是因为,当Al粉含量少于理论化学计量比的复合物时,虽然点火端升温较快,但由于放出的热量小,即式(2)中H较小,使得发生温度突跃,计算不再收敛的时间(即点火时间)增大。然而当Al粉含量增大时,点火阶段升温速率和放出的热量H均逐渐减小,导致点火时间增加。因此,综合Al和Bi2O3的配比对反应放热、密度、热容、热扩散速率等因素的影响,点火时间最短的反应物配比为2。另一方面,随着反应物配比的增大,反应物的热容逐渐增大,综合单位质量放热焓H的影响,反应的平衡温度逐渐减小。由此可见,反应物的单位质量放热焓对点火时间影响作用较为明显,而反应平衡温度受反应物热容的影响较为明显。
图6显示了反应产生的燃烧波传播速度和平均反应速率随反应物配比的变化关系。对于摩尔配比为1.5、1.8、2、2.5、3、4的复合物,燃烧波传播速度V分别为93.8 m/s、119.25 m/s、671.4 m/s、601 m/s、408.3 m/s和263.2 m/s,平均最大反应速率φmax分别为9.28 × 105 s−1,5.25 × 106 s−1、1.37 × 107 s−1,1.06 × 107 s−1、8.12 × 106 s−1和4.88 × 106 s−1。从图6可以看出,Al和Bi2O3配比为2的Al/Bi2O3具有最大的燃烧波传播速度和反应速率。这同样因为,在装药量固定的条件下,反应物配比为2的复合物放出的热量最多,即对应热扩散方程中的H最大。
由以上对比可知,当Al/Bi2O3复合物摩尔配比为2时,各种燃烧行为达到最佳,Al的不足和过量都会影响其燃烧行为,尤其是当Al含量不足时,燃烧波传播速度和化学反应速率减少更加明显。

Figure 4. Heating curves of the ignition with different mole ratio of Al/Bi2O3
图4. 不同反应物配比点火端的温升曲线

Figure 5. Ignition delay time as a function of mole ratio of Al/Bi2O3
图5. 反应物配比对反应点火时间的影响
 (a)
(a) 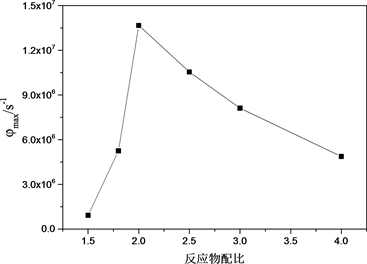 (b)
(b)
Figure 6. Propagation velocities of combustion and maximum rates of reaction as functions of mole ratio of Al/Bi2O3. (a) Propagation velocities of combustion; (b) Maximum rates of reaction
图6. 反应物配比对燃烧波传播速度和最大反应速率的影响。(a) 燃烧波传播速度;(b) 最大反应速率
3.2.2. 粒径
Al粉粒径分别为2 μm、100 nm、40 nm、24 nm的Al/Bi2O3复合物反应区温度、反应速率、燃烧波传播速度、点火时间随粒径的变化关系如表2所示,点火端的温升曲线如图7所示。
由表2可以看出,粒径为2000 nm的Al/Bi2O3复合物具有最大的反应平衡温度。这是因为粒径为2000 nm的复合物具有较高的反应活化能,导致点火时间较长,在燃烧前升温阶段输入的热量较多,导致平衡温度略有增加。随着粒子粒径的减小,反应区达到局部平衡时的温度相差不大,但反应过程中的反应速率和燃烧波传播速率明显增大。粒径的减小主要影响反应的活化能和指前因子,因而粒径的减小可以明显地提高燃烧性能,即增大反应速率和燃烧波传播速度。
然而,当粒径由40 nm变为24 nm时,虽然最大反应速率与燃烧波传播速度仍保持增大的规律不变,但反应的点火时间反而略有增加。这是因为粒径为24 nm的实际测得的活化能要高于40 nm复合物,Aumann等 [14] 将活化能增大的这一现象归因于在实验材料制备过程中出现的无定型铝晶体。江自生 [17] 在研究中也发现了这一现象,当Al/Bi2O3复合物粒径由96 nm变为53 nm时,反应压力和能量释放速率虽然增大,但反应点火时间也增大了。
以上计算及文献数据对比表明,纳米级粒径的MIC复合物燃烧性能较微米级粒径的MIC材料有很大幅度的提高,然而当粒径减少到一定程度,由于无定型晶体形貌的形成,继续减小粒径会导致活化能略有增加,反应点火时间变大,但不影响能量释放率的增强,即反应点火时间受到反应物的活化能的影响,且影响作用较化学反应的能量释放更明显。
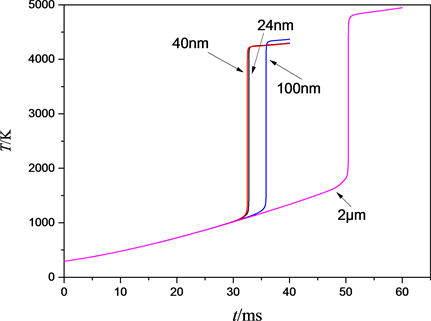
Figure 7. Heating curves of the ignition with different particle size
图7. 不同铝粉粒径材料的点火特性

Table 2. Results in simulation of combustion characteristics of Al/Bi2O3 with different particle size
表2. 不同粒径Al/Bi2O3复合物温度特性计算结果
4. 结论
本文从MIC材料热扩散和热传导角度建立了燃烧特性数值计算的物理与数学模型,计算分析了Al/Bi2O3典型MIC材料的燃烧特性,并研究了反应物配比、粒径等对Al/Bi2O3材料反应的影响,得到了以下结果。
1) Al/Bi2O3温度计算结果表明,MIC材料在反应过程中经历了三个阶段,即点火前升温阶段、燃烧波传播阶段和局部热力学平衡阶段。
2) 由MIC材料反应影响因素计算结果可知,反应物配比为2的Al/Bi2O3复合材料反应性能最好,平衡温度可达4217 K,平均最大反应速率和燃烧波传播速度分别为1.36 × 107 s−1和671.6 m/s。
3) 随着反应物粒径的减小,Al/Bi2O3的平衡温度逐渐减小,最大反应速率和燃烧波传播速度逐渐增大,反应物的活化能是影响反应点火时间的重要因素,且影响作用较化学反应的能量释放更明显。
基金项目
爆炸科学与技术国家重点实验室(北京理工大学)自主课题资助项目(YBKT16-06)。
NOTES
*通讯作者。