1. 引言
随着超分子化学的发展,分子识别在合成化学、生命科学、信息科学以及材料科学等领域中起着越来越重要的作用。分子识别是指分子之间通过非共价键结合而形成特定功能体的过程 [1] [2] [3] 。为了使分子识别过程所包含的信息简单有效的向外界传递,可通过巧妙设计的具有分子器件性质的光化学传感分子所表现出来的光学信号来传递。这些光化学传感分子被称为光化学受体或者光化学传感器(光化学探针)。光化学传感分子包括比色传感分子和荧光传感分子,比色传感分子可以通过颜色变化来实现目标分子的检测,这种方法简单,不需要贵重仪器,是一种“裸眼”识别方法;而荧光传感分子是通过荧光的变化来实现对目标分子的识别,该方法选择性高、灵敏度高、检测限低,易于实现细胞原位检测和在线分析 [4] [5] 。
阴离子识别与传感是当前超分子化学研究领域一个重要和活跃的研究课题,与生命过程有关的阴离子的光化学传感体系已经引起了广泛关注,与之相关的合成受体设计备受关注。酰胺、胺、硫脲、脲、胍、吡咯和酚羟基等具有氢键作用的基团作为阴离子结合单元,已被广泛应用于合成受体设计和阴离子识别研究,而具有荧光/吸收光谱响应的信号报告基团的引入赋予受体良好的光化学传感性能 [6] [7] [8] [9] 。
作为一类重要的有机荧光染料,氟硼荧(BODIPY, 4,4-difluoro-4-borata
-3a
-azonia
-4a
-aza-s-indacene)类化合物及其荧光探针研究倍受关注。与被用作荧光载体的色素和其他稠环芳烃相比,BODIPY类荧光染料具有高摩尔消光系数、高荧光量子产率、最大激发波长可达到500~700 nm、结构易修饰、不易受环境和溶液pH值的影响、光化学稳定性良好等优点。通过分子设计,有效组合各种不同的活性单元制备的功能化BODIPY类荧光探针已应用于质子、金属离子、阴离子、有机分子和气体分子检测。DNA的标记和测序以及生物体系中的单糖,类脂膜和蛋白质等的动力学、结构和功能研究 [10] [11] [12] 。在前期的研究基础上,设计合成了含有酚羟基结构的BODIPY基荧光受体,针对合成的受体1,利用紫外可见吸收光谱,荧光发射光谱考察了传感分子1的阴离子识别和传感性能,并探索了其识别作用和传感机制。
在普通高校的化学化工、药学和农学等专业的仪器分析教学中,紫外–可见吸收光谱法和荧光发射光谱法占据了重要地位,这得益于其在分析化学领域中极其广泛的应用价值。现行的大学分析化学实验基本上采用传统的验证性实验教学模式,通常是采用分光光度计测定一个物质的线性范围,然后测定该物质的浓度。虽然对培养学生的实验操作技能及数据处理技术等有显著效果,但这种机械式模仿使学生在整个教学环节中处于被动学习的地位,使实验教学的启发性和探索性功能受限,无法培养学生的独立思考能力、创新能力和创新精神。基于此,我们将科学研究中具有良好光谱性能的荧光探针的研究引入到本科实验教学中来,一方面可以激发学生的科研热情,另一方面可以让学生深入学习仪器的使用方法,为将来的科研和工作打下基础。
2. 实验目的
1) 了解分子识别与光化学传感以及荧光探针的基础知识。
2) 设计合成一类可以用于阴离子识别与传感研究的简单荧光探针分子。
3) 通过实验研究探针分子的阴离子识别与传感过程的实验现象和机理,掌握紫外–可见吸收光谱和荧光发射光谱仪的使用方法。
3. 实验原理
该实验设计合成了一类阴离子光化学探针分子,酚羟基作为阴离子的识别位点,可以与阴离子之间形成氢键,通过氢键作用引起紫外可见吸收光谱和荧光发射光谱的变化,进而达到分子识别和光化学传感的目的。
4. 仪器与药品
4.1. 实验仪器
Lambda 35型紫外可见分光光度计(美国PE公司),LS 55型荧光光度计(美国PE公司),Inova 400 MHz型核磁共振仪(美国Varian公司),Agilent 1100 LC/MSD Trap型液质联用仪(美国Agilent公司),81-2型恒温磁力搅拌器(上海司乐仪器有限公司)。
4.2. 实验试剂
水杨醛,2,4-二甲基吡咯,3,5-二硝基苯甲酰氯,二氯甲烷,氯仿,乙醇,甲醇,丙酮,正己烷等分析纯试剂,四丁基氟化铵(TBA-F),四丁基氯化铵(TBA-Cl),四丁基溴化铵(TBA-Br),四丁基碘化铵(TBA-I),四丁基硫酸氢铵(TBA-HSO4),四丁基醋酸铵(TBA-AcO),四丁基磷酸二氢铵(TBA-H2PO4),四丁基氢氧化铵(TBA-OH),四丁基高氯酸铵(TBA-ClO4),乙醇和CH3CN等均为分析纯试剂(阿拉丁试剂公司); CH3CN-d6 (Cambridge Isotope Laboratories, Inc. USA)。
5. 实验方法
5.1. 探针分子1的合成与表征
探针分子1的合成路线如图1所示,化合物2在前期的工作中已被合成 [13] 。
1 (C33H29BF2N4O2)的合成方法:取2 (0.35 g, 1 mmol)溶于EtOH/CH3CN (5:1, v/v, 50 mL),加热使其全部溶解,加入过量水杨醛(0.37 g, 3 mmol),滴加3滴冰醋酸作为催化剂,加热回流4 h,冷却至室温,有黄色固体物质析出,将其过滤出来,无水乙醇洗涤三次,干燥后得到纯净的化合物1,产率70%。
HR MS: C33H29BF2N4O2, Anal. Cal. M + H = 563.2424; Found 563.2430; error = 1.1 ppm.
1H NMR (CH3CN-d6, 400 MHz): 12.85 (s, 2H, OH), 9.14 (s, 2H, −CH=N), 7.65~7.70 (m, 3H, ArH), 7.41~7.48 (m, 4H, ArH), 6.97~7.01 (m, 4H), 6.22 (s, 2H, ArH), 2.47~2.50 (m, 6H, −CH3), 1.54 (s, 6H, −CH3).
5.2. 荧光探针标准溶液和阴离子溶液的配制
准确称量探针分子1,将其配制成1.5 × 10−4 mol/L的CH3CN溶液,备用。
准确称量TBA-F,TBA-Cl,TBA-Br,TBA-I,TBA-HSO4,TBA-AcO,TBA-H2PO4,TBA-OH,TBA-ClO4,
配制上述9种物质的1.5 × 10−2 mol/L和1.5 × 10−3 mol/L的CH3CN溶液,备用。
5.3. 紫外–可见吸收光谱与荧光发射光谱的测定方法
移取1 mL探针分子1的CH3CN溶液(1.5 × 10−4 mol/L)于一系列10 mL比色管中,分别向比色管中加入10倍的阴离子(F−, Cl−, Br−, I−, AcO−,
,
,
),然后将其稀释10倍至探针分子浓度为1.5 × 10−5 mol/L,重复震荡后混匀,在室温下测试探针分子与不同阴离子作用的紫外–可见吸收光谱和荧光发射光谱。
在测试探针分子1对氟离子的光谱滴定实验时,采用累积加样法:固定探针分子的浓度为1.5 × 10−5 mol/L,采用微量注射器加入0~10倍的氟离子,然后在室温下测试其紫外–可见吸收光谱和荧光发射光谱。
6. 实验结果与讨论
6.1. 探针分子与阴离子作用的紫外–可见吸收光谱
首先考察了CH3CN溶液中探针分子1与各种阴离子(F−, Cl−, Br−, I−, AcO−,
,
,
)相互作用的紫外–可见吸收光谱变化。
受体1的CH3CN溶液在342和499 nm处有两个吸收峰,当与不同阴离子作用时,只有氟离子可以引起紫外–吸收光谱的变化,在435 nm左右出现了一个新的吸收峰(如图2)其他离子的加入并不能引起紫外–可见吸收光谱的明显变化。氟离子存在下所产生的吸收峰与强碱[TBA]OH所引发的光谱变化是不同的,说明受体1与氟离子作用是氢键作用,没有发生去质子。在氟离子存在下,往体系中加入少量水之后,可以恢复到初始状态,进一步说明氟离子与受体之间的氢键作用本质。
为了研究1与氟离子之间的作用,然后进行了1对氟离子的紫外光谱滴定实验。图3为受体1与氟离子作用的紫外滴定图,从图中可以看出,受体1在342和499 nm处的吸收峰逐渐降低,在435 nm处出现一个新的吸收峰。在滴定过程中,在375 nm处出现了等吸点,说明只有一种物质产生。
6.2. 探针分子与阴离子作用的荧光发射光谱
如图4为探针分子1与阴离子作用的荧光发射光谱图,1在510 nm处有一个荧光发射峰,当探针分子与不同阴离子作用时,只有氟离子可以导致受体发射光谱的明显变化,加入10倍当量的氟离子使荧光强度降低9倍,而其他阴离子引起的发射光谱的变化较小。

Figure 2. Changes in the UV/vis absorption spectrum of 1 (1.5 × 10−5 mol/L) in CH3CN solution upon addition of 10 equiv. of various anions (F−, Cl−, Br−, I−, AcO−,
,
,
)
图2. 探针分子1 (1.5×10−5 mol/L)在CH3CN溶液中与10倍不同阴离子(F−, Cl−, Br−, I−, AcO−,
,
,
)作用的紫外–可见吸收光谱变化图

Figure 3. Changes in the UV/vis absorption spectrum of 1 (1.5 × 10−5 mol/L)in CH3CN upon addition of F− anions in TBA salts form ( 0 - 20 equiv.)
图3. 探针分子1 (1.5 × 10−5 mol/L)在CH3CN溶液中与F− ( 0~20 倍)作用的紫外–可见吸收滴定光谱
图5为探针分子1对氟离子的荧光滴定图。从图中可以看出,随着氟离子的加入,荧光发生持续淬灭,受体的溶液绿色荧光逐渐消失。由于氟离子的加入,诱发了受体1的PET (photo-induced electron transfe,光诱导电子转移)过程 [14] ,这是受体荧光淬灭的原因。研究表明探针分子1是一类氟离子的开–关型荧光探针。
6.3. 探针分子1与氟离子之间的结合配比及结合常数
主客体之间的结合常数可以通过Benesi-Hildebrand方程 [15] 来计算。
图6为探针分子1的线性拟合图。探针与氟离子之间的结合配比为1:1,结合常数K计算结果为1.1 × 103
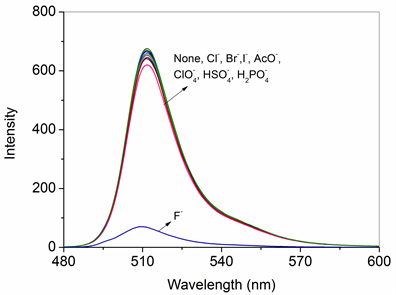
Figure 4. Changes in the emission spectrum of 1 (1.5 × 10−5 mol/L) in CH3CN upon addition 10 equiv. of various anions (F−, Cl−, Br−, I−, AcO−,
,
,
) (λex = 490 nm, slit = 5/5)
图4. 探针分子1 (1.5 × 10−5 mol/L)在CH3CN溶液中与10倍不同阴离子(F−, Cl−, Br−, I−, AcO−,
,
,
)作用的荧光发射光谱变化图(λex = 490 nm, slit = 5/5)
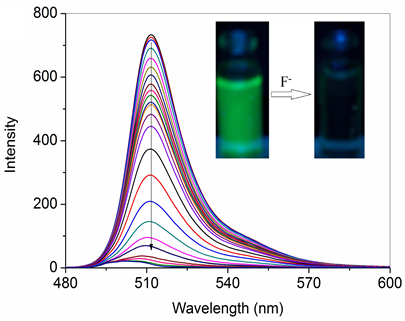
Figure 5. Changes in the emission spectrum of 1 (1.5 × 10−5 mol/L)in CH3CN upon addition of 0 - 5 equiv. of F−
图5. 探针分子1 (1.5 × 10−5 mol/L)在 CH3CN溶液中与F− ( 0~5 倍)作用的荧光滴定光谱
M−1,线性相关系数为0.991。
根据前面的分析结果,可以将受体1与氟离子作用方式以图7的形式表现出来。从图中可以看出,受体1与氟离子通过氢键形成1–F−复合物,诱发了荧光受体的PET过程,从而导致受体荧光淬灭。
7. 结论
设计合成了含有酚羟基的BODIPY基化合物1,通过紫外–可见吸收光谱和荧光发射光谱手段研究了探针分子1与阴离子作用方式和传感机制。探针分子1是一类开–关阴离子荧光探针,作用方式为氢键作
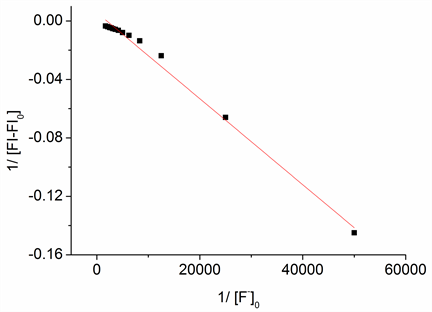
Figure 6. Benesi-Hildebrand plot assuming 1:2 stoichiometry for association between 1 and F−
图6. 探针分子1与氟离子之间的线性拟合图

Figure 7. Proposed interaction mode of sensor 1 with fluoride anion
图7. 探针分子1与氟离子作用机理图
用,光诱导电子转移(PET)是探针分子荧光淬灭的原因。本实验综合了有机合成和结构表征、光化学分析测试仪器的使用操作等多方面的知识,为进一步深入研究荧光探针的合成和应用奠定了基础。作为高校教师,在仪器分析的教学过程中,应该站在时代前沿,将科学研究的内容引入到实验教学中,可以有效提高学生学习的兴趣和动力,理论联系实践,融会贯通,全面发展。
致谢
感谢甘肃农业大学学生科研训练计划项目和甘肃农业大学“伏羲青年新秀”人才引进项目资助。
基金项目
甘肃农业大学学生科研训练计划项目(SRTP20170103&20170104)和甘肃农业大学伏羲青年人才引进项目。