1. 引言
众所周知,小麦种子由皮层、胚、胚乳组成。糊粉层分布在胚乳的最外层,胚与糊粉层细胞均为活细胞 [1] 。胚一般占种子质量的2%~3%,糊粉层约占种子质量的7%,胚乳大约占种子质量的90%~93%。成熟脱水的小麦种子含水量较低,在种子检验标准中,不超过13%为合格的含水量 [2] 。低水分对种子细胞膜会产生不良影响。在水分含量降低的过程中,种子的膜失去水分,为确保磷脂双分子层表面氢键的形成,富含羟基的糖类 [3] [4] 、脯氨酸、两亲蛋白 [5] [6] 等替代水分聚集在膜表面,与磷脂发生相互作用 [7] ,维持膜的结构,减少脱水过程对细胞的损伤。随着脱水膜由溶液态转变为中间态 [8] ,进一步降低水分,低于5%还可以转变为玻璃态 [9] ,仍不失去活性,如果此时变为较硬的凝胶态细胞将失去活性 [10] 。细胞随着脱水逐渐从溶液态到粘液态、再到橡胶态、皮革态,最后是玻璃态 [11] 。无论哪种状态,细胞都可以是活的,但膜完整性存在细微差异 [11] 。
吸胀过程中种子内含物会大量渗出,豌豆种子由胚外的细胞最先渗出电解质 [12] 。电解质和有机物都可以渗出 [13] ,无机离子最常用的检测方法是浸泡液的电导率,电导率是代表质膜透性变化最常用指标之一 [14] [15] ,而对有机物,检测方法较多 [12] ,鲁黎明 [16] 等用260 nm和280 nm紫外吸光度法、Tai Gi Min [17] 用刃天青变色来表示,总之用紫外吸光度检测的较多。紫外吸光度与电导率检测相似,检测的是总有机物的量,不是针对某种物质进行的。种子浸出液中电导率和吸光度的大小在一定程度上反应种子内容物质外渗情况 [18] 。外渗量的多少会影响到种子的活力 [13] 。
种子活力测定的经典方法之一即通过种子浸泡液的电导率来反应种子活力 [18] [19] 。电解质渗出量越高,种子的活力越低,二者呈负相关关系。然而,影响电导率的因素颇多,通过24 h的浸泡,无法判断期间种子的具体变化,TTC染色可以看出颜色变化 [20] ,但是无法判断种子哪一部分渗出的物质较多,种子的膜系统修复发生在哪一时间段?是因为淹水缺氧还是因为膜修复慢导致了电导率的升高?
细胞学的观察则可以对不同部位的细胞透性进行比较。荧光染料PI (碘化丙啶Propidium Iodide)是一种可对细胞核中DNA染色的荧光染色试剂,常用于细胞凋亡检测,在嵌入双链DNA后释放红色荧光 [21] 。尽管PI不能通过活细胞膜,但却能穿过破损的细胞膜而对核染色,通过活体切片的PI染色和荧光染色观察。可以了解不同部位细胞通透性的变化,对我们了解干种子膜的状态有一定的参考价值。
2. 材料与方法
2.1. 种子及去胚处理
供试品种为潍麦8号、邯郸6172,购自潍坊市种子销售点。潍麦8号是2003年通过山东省审定的高产、抗病、耐肥水小麦品种,邯郸6172是生产上的主推品种之一。将破损、霉变及畸形小麦种子剔除出去。去胚处理为将种子胚用小刀小心挖出,然后用502胶水将缺口封住。
2.2. 老化
根据种子含水量测定标准方法烘干法 [2] ,测出各样品的含水量,各取50 g种子分别加入分别4.69 g和5.75 g水,而后室温下充分混匀,使两种种子的含水量达到20%,用封口机把小麦种子封在塑料袋里,放在恒温箱中。42℃的劣变处理6 d。
2.3. 标准发芽试验与发芽指数的测定
用1%次氯酸钠溶液浸泡小麦种子5~8 min,然后用蒸馏水冲洗3~5次,按照标准发芽试验方法进行发芽率测定,4次生物学重复,在智能光照培养箱中,日温25℃,8 h,照光,夜温20℃ 16 h。每天统计发芽数,进行发芽指数的计算,发芽势以发芽第4 d的发芽率计算 [2] 。
2.4. 种子相对电导率的测定和渗漏有机物相对含量的测定
称取每个样品种子2 g (约50粒),三次重复,去胚种子、老化去胚种子称重后进行去胚和封口处理。放入小塑料网中,迅速用纯净水冲洗,用滤纸吸干种子表面的水,然后分别放在含有50 ml纯净水的烧杯中,20℃浸泡,每隔1 h倒出浸泡液摇匀后进行电导率和260 nm处吸光度检测分析,检测后再倒回去,直至6 h。完全透性对照种子加入50 ml蒸馏水后先放在沸腾的水浴锅中煮7 min,使种子完全丧失活力,然后将浸泡液倒出,测量体积,用纯净水补齐至50 ml,以后的浸泡和测量同其他种子。将6 h的总渗出量作为100%渗出量。
2.5. 数据统计分析
相对电导率的计算:以水煮死亡种子电导率为完全透性电导率,为100%,样品测定的电导率除以该种子完全透性电导率为该种子的相对电导率。发芽率、发芽指数和发芽势的统计分析和比较采用文献22的方法进行 [22] 。
2.6. 种子胚与胚乳的PI染色透性检验
冰冻切片的制作:采用日本樱花Tissue-Tek Cryo3冰冻切片机和奥林巴斯荧光显微镜,提前半小时开启冷冻切片机,冷冻室温度−30℃,等温度降下来后,设置切片厚度17 μm,在冷冻室的样品台涂一层OCT包埋剂,将含胚的小半粒干种子放入包埋剂中冷冻至成白色,装上样品,进行切片,载玻片上每粘贴一张切片后即观察,结构完整的进行染色,用移液器吸取10 μg∙mL−1 PI试剂工作液,进行染色,每个切片约1~2滴,盖上盖玻片,避光条件下染色25~30 min,染色完成后在荧光显微镜下镜检。分别在4倍目镜和10倍目镜下观察并拍照,以内标尺作为放大倍数的参考。
3. 结果与分析
3.1 老化处理后种子的发芽指标变化
两个品种中潍麦8号是2016年的种子,邯郸6172是2014年低温保存的种子,从表1标准发芽试验可知,种子的发芽率均较高。发芽势和发芽指数是种子活力的相关指标。经过老化处理后,两个品种的发芽率、发芽势和发芽指数下降显著,活力下降也显著,处理后的种子只有约80%的发芽率,发芽势下降了17.2%~18.2%,两品种有相似的变化趋势,经过老化处理均达到了种子活力差异显著,多数种子依然存活的目的。
3.2. 胚乳与完整种子浸泡液相对电导率的变化分析
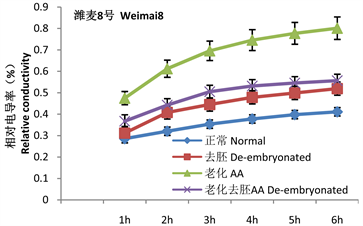
2 g干种子胚的平均重量为0.02 ± 0.0052 g。重量小,又有伤口,无法直接测量胚的膜透性,只能间接测定完整种子和去胚种子的膜透性来推算。通过相对电导率的计算可以看出(图1),种子相对电导率变化的趋势是在第1 h内渗出量最大,以后随着时间的推移,渗出量增量逐渐减少,活种子和死亡种子渗出量增量均减小。第1 h的渗透量是变化最大的,也是样品间差异最明显的时间段。
 (a)
(a) 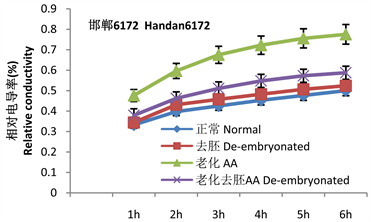 (b)
(b)
Figure 1. Changes in relative conductivity of seeds and endosperms of the two varieties
图1. 两品种种子与胚乳相对电导率的变化标准试验系统结果曲线
表1. 两品种老化处理后发芽指标的变化
注:A、B字母表示统计分析显著性水平0.01。Note: The letters A and B indicate that statistical analysis difference at level of 0.01 is significant.
从相对电导率可以看出老化处理使种子渗透量显著升高,两个品种未经老化的高活力种子渗透量分别占总渗漏量的28%和33%,老化后两品种均升高到45%。两个品种老化去胚种子第1 h分别有37%和38%渗出量(包含未完全封闭的伤口的渗出量),胚在老化处理后的吸胀1 h电解质渗出量增幅分别为7%和8%。这些增量应该是胚的渗出量。
种胚的电导率需要通过正常种子和去胚种子相比电解质的减少量表示,胚本身较小,如果胚的离子渗出较少,应具有与干种子相似的电导率。但未经老化的种子去胚后电导率却略大于正常种子的电导率。说明在处理过程中胶并不能完全封闭住伤口,去胚后不但未减少,反而略微升高。说明上面计算的增量值是偏低的,实际应该包括未封闭住伤口导致的误差。
3.3. 胚乳与完整种子有机物渗透量分析
对于简单的公式,小麦种子浸泡液渗出量的全波长扫描分析结果见图2,从图2可知,种子由于渗漏的可发生光吸收的物质是混合物,因而图上没有特定波长下的吸收峰,随着波长的增长吸光度逐渐减少,不同浸泡时间下的曲线吸收值不同,250~280 nm是相对吸收量变化较平稳的区间,在此区间内选择260 nm作为有机物的检测波长进行有机物的相对含量检测。
根据表2可知,以死种子6 h的渗透量为100%计算。两个品种不同处理在第1 h内的相对渗出量都比较高,说明浸泡的第1 h种子内的有机物大量渗出,随着时间的推移,种子浸泡液总有机物渗出量不断增加,但后期变化缓慢。
老化和正常种子间的渗出量差异是显著的。两个品种的老化及去胚处理种子渗透量的变化趋势存在一定差异,潍麦8号变化趋势与电导率相似,即老化和老化去胚的渗出量差异是显著的,在第1 h经老化处理,与正常种子相比,老化种子相对渗漏量增加了11.4%,说明老化使相对透性提高了,而老化去胚即仅有胚乳的种子相对渗出量为49.29% (包括由于缺口未完全封闭而造成的损伤泄漏)。由此可以推算出,
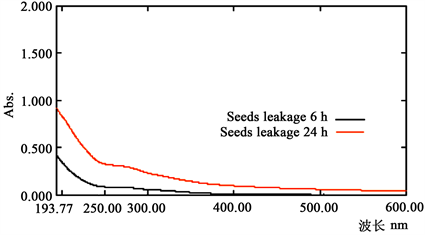
Figure 2. Changes in light absorption of wheat seeds at different wavelengths
图2. 小麦种子在不同波长下光吸收的变化
表2. 浸泡液吸光度在第1 h的相对变化
注:a、b字母表示统计分析显著性水平0.05。Note: The letters a and b indicate that statistical analysis difference at level of 0.05 is significant.
在第1 h渗透增量约有5%是由胚引起的。胚的重量远远小于胚乳,50粒胚重量约0.025 g,说明相同体积水中胚用远小于胚乳的重量提供了至少5%的渗透增量。邯郸6172老化与老化去胚相比,渗漏有机物的增量并不高,约4%,但正常种子的渗漏量较小。
3.4. 胚与胚乳透性的解剖学观察
从前面的结果可以看出,干种子的细胞膜是不完整的,可以透过大量的有机物和电解质,然而我们并不清楚胚和胚乳哪里的选择透过性高。PI在膜具有选择透过性的前提下是不能透过细胞进入核的,但如果细胞膜有损伤或伤口,PI就可以进入。从图3我们看出,干种子的胚乳及胚外层细胞与经过FAA固定后没有选择透过性的死亡组织具有相似的红色荧光亮度,说明干种子的胚乳以及胚组织外层细胞均透性较高。胚乳组织可染色的有核细胞密度较小。胚根和胚芽部位荧光亮度明显下降,而死亡的胚根和胚芽则亮度极高。说明尽管是干种子,胚部位的选择透过性依然较好。
从图4可以看出,经过劣变老化处理后,胚与胚乳都具有较强的透性,PI可以透过胚根及胚其他部位,从而显出较强的红色荧光。胚内部的红色荧光强度不及外围细胞,说明具有一定的选择透过性。胚内细胞密度大,细胞较小,细胞核清晰可见。
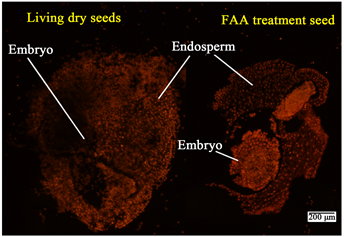
Figure 3. PI dyeing of dry seeds of normal wheat and fixed dead seeds. Note: The bar in the figure means ruler of 200 μm in the figure. Living dry seed refers to the living seeds embryos, and FAA treatment seed refers to the seeds that are dead after the FAA fixed
图3. 正常小麦干种子与固定后死种子的PI染色。注:图中的杆表示200 μm标尺。图中Embryo指胚,Endosperm指胚乳,living dry seed指活的种子,FAA treatment seed指经过FAA固定后死的种子
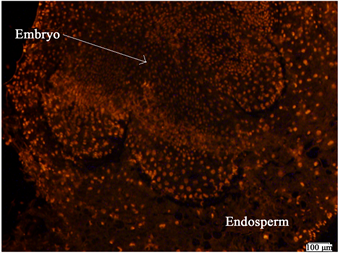
Figure 4. PI staining of embryo and endosperm of wheat seeds after AA treatment. Note: The bar in the figure means ruler of 100 μm in the figure
图4. 老化处理后小麦种子的胚与胚乳的PI染色。注:图中的杆表示100 μm标尺
4. 讨论
4.1. 小麦种子吸胀初期膜透性变化规律
干种子是较为特殊的生命状态,种子是活着的,细胞却又是不活跃的,Stanley H [23] 报道在无种皮的条件下渗出的大分子较多,而有种皮的种子则很少渗漏出大分子。说明种皮障碍可以阻止大分子的随机扩散。因此通过加热处死种子并不是所有物质都可以从细胞内向外渗漏。采用相对电导率和相对有机物的量可以更好地反应种子的细胞透性。
本研究发现在浸泡的第1 h内渗漏的物质最多,J. Brad [24] 认为干种子膜一旦吸水立即变成有组织的结构,有选择透过性,David J. [25] 认为大豆种子也是在吸胀的最初几分钟渗漏量最大,在30 min以内呈线性变化,因此本研究在分析数据时主要分析浸泡1 h的渗漏量。数据结果显示1 h浸泡液不同活力或处理间有显著差异,活力低的种子渗漏量(电导率、紫外检测值)远高于活力高的,且胚对数据的升高贡献较大,与活力有关。
4.2. 胚和胚乳在吸胀初期膜透性的比较
普遍认为干燥的种子膜系统是不完整的,然而磷脂双分子层依然是存在的 [11] 。目前并没有明确有效的方法来比较小麦在吸胀过程中胚和胚乳的透性。本研究对正常种子去胚处理会在胚表面造成大量破损细胞,可导致物质外渗,但无法直接测量。由于去胚以后的胚乳损伤面积与整个种子比相对较小,可以通过封阻的方法抑制损伤细胞部位的物质外渗,因此可检测胚乳的渗漏量。但封阻方法尚不成熟,会导致部分去胚处理测量结果不准确,胚乳的渗出量有时会高于总渗漏量。这可能是胚乳破损细胞封闭不严格,损伤细胞物质外渗导致。通过增加试验次数可以尽可能缩小误差。但很难知晓胚的具体贡献。当正常种子进行老化处理后,种子活力显著下降 [26] ,物质外渗总量显著增加 [27] 。增加的渗透量应由活细胞引起,胚组织基本都是活细胞。把老化处理的种子去胚后测量其吸光度或电导率,发现明显小于老化种子,且与未老化去胚的种子相差较小。说明吸光度或电导率的增量主要贡献者是胚,间接说明老化的种子胚膜透性显著增强。
4.3. 胚组织PI透性检测
上文中计算的胚透性并不是直接的证据,还有必要进行直观的观察。PI是细胞不能选择透过的物质 [21] ,只要进入细胞就说明细胞某些部位可以失去选择透过性。透过得越多,在荧光显微镜下红色荧光越强 [28] 。通过调节荧光强度和曝光时间,干种子切片上所有的细胞都可以显示出荧光,只是透过的多少有别。死种子不具有选择透过性,PI可以自由出入,进行透性检测时,在同一视野内既有死种子,又有活种子,可以比较活种子的透过能力。本研究认为干种子的胚,尤其是胚根、胚轴、和胚芽等为关键部位,PI的透性明显降低。说明活的胚组织细胞结构显著好于其他部位细胞。当胚活力降低时,其透性与其他细胞的差异就很小了,这可能是胚活力降低的原因之一。
致谢
感谢山东省自然基金对本项目的支持,感谢潍坊市农业科学院魏秀华研究员对本课题的品种推荐和支持。
基金项目
山东省自然科学基金(ZR2014JL017),潍坊市科技发展计划(20111117)。