1. 引言
Nudel是一类进化上保守的蛋白,在体内参与多个信号通路的调控与疾病的发生。Nudel与多种蛋白质相互作用,包括dynein、lissencephaly 1蛋白(Lis1)、精神分裂症1 (Disc1)和
14-3-3
ζ [1] [2] [3] 。Eva Klinman等人的研究显示,Nudel/Lis1复合体通过直接调控动力蛋白的活性,激活异常的CDK5,进而引起神经轴突的运输中断,而轴突运输的中断与很多神经退行性疾病有关 [4] 。Moon等人的研究表明,Nudel集中于着丝粒附近,调控有丝分裂纺锤体的形成和有丝分裂的过程 [5] 。另外,Nudel具有寡肽酶活性,有望作为潜在的精神分裂症的生物分子标记 [6] 。Nudel可以与动力蛋白相互作用而影响细胞运动和物质运输,例如溶酶体运输 [7] 和细胞器的定位 [8] 。Nudel也可以不依赖细胞质动力蛋白而参与细胞迁移,它通过与Cdc42竞争结合Cdc42GAP,使Cdc42从Cdc42GAP上释放而被活化,从而阻止细胞的极化与伪足的形成。除此之外,Nudel通过与黏着斑蛋白桩蛋白相互作用,而有助于在迁移细胞的前缘形成新的吸附 [9] 。细胞迁移与肿瘤转移密切相关,Pten是著名的肿瘤抑制因子,很多研究表明,Pten是细胞运动的负调控因子 [10] [11] ,在Pten突变体中,细胞运动和增殖会加剧,这是细胞癌变的标志 [12] 。Pten是细胞骨架的通用开关,通过调控肌动蛋白和微管防止细胞过度增殖 [13] 。本研究以A549细胞为材料,进行Nudel基因的克隆、鉴定及在COS7细胞中的表达等,并初步检测了抑癌基因Pten对Nudel表达的影响,为进一步研究Nudel基因对及细胞迁移的影响打下基础。
2. 材料与方法
2.1. 材料
A549细胞、pDsRed-C1载体、感受态细胞DH5α由本实验室保存;pMD18-T、T4连接酶购自TaKaRa公司;内切酶Xho I、BamH I购自Thermo公司;胶回收试剂盒:E.Z.N.A Gel Extraction Kit(100)试剂盒;质粒提取试剂盒:E.Z.N.A. Plasmid Mini Kit I(100)试剂盒;超纯质粒提取试剂盒:E.Z.N.A Endo-free Plasmid Mini II试剂盒,Trizol、逆转录试剂盒M-MLV购自Invitrogen公司;转染试剂PEI购自Proteintech公司。
2.2. RT-PCR扩增Nudel目的基因
选取生长状态良好的A549细胞(培养条件:RPMI 1640培养基,10%胎牛血清,37℃,5% CO2)为材料,提取总RNA。以此RNA为模板,参照Invitrogen的说明书进行反转录获得cDNA。以cDNA为模板,扩增目的基因Nudel,扩增体系:ddH2O 15.5 μL,cDNA 0.5 μL,上下游引物各0.5 μL,dNTPs (
10 mM
) 2 μL,10 × EXTaq Buffer,EXTaq 0.5 μL。扩增条件:预变性95℃ 5 min,95℃ 30 s,50℃ 30 s、72℃ 1 min 30 s,35个循环,72℃ 10 min,最后4℃保存。扩增Nudel的引物,上游引物:
5’
-ATGGATGGTGAAGATATACCAG
-3’
,下游引物:
5’
-TCACACACTGAGAGGCAGCATAC-3’(引物自行设计,由武汉擎科公司合成)。
2.3. 构建pT-Nudel和pDsRed-Nudel载体
将扩增的目的基因克隆至pMD18-T载体上,进行测序鉴定,正确的克隆命名为pT-Nudel。以测序鉴定后的质粒pT-Nudel为模板,采用高保真酶扩增Nudel目的基因。扩增体系:ddH2O 34 μL,pT-Nudel质粒DNA稀释100倍取1 μL (约50 ng),上下游引物各1 μL,dNTPs (
2 mM)
5 μL,MgSO4 (
25 mM)
2 μL,10 × KOD buffer 5 μL,KOD Polymerase 1 μL。扩增条件:预变性95℃ 5 min,95℃ 30 s,50℃ 30 s、68℃ 1 min 30 s,35个循环,68℃ 10 min,最后4℃保存。扩增Nudel的引物,上游引物:
5’
-CCGCTCGAGATGGATGGTGAAGATATACCAG
-3’
,引入Xho I酶切位点,下游引物:
5’
-CGGGATCCTCACACACTGAGAGGCAGCATAC
-3’
,引入BamH I酶切位点。
2.4. Nudel基因在真核细胞中COS7中的表达
制备去内毒素的超纯pDsRed-Nudel质粒,检测DNA的浓度与纯度,转染到COS7细胞,并在荧光显微镜下进行观察。细胞转染:采用PEI转染试剂进行转染,12孔板种细胞过夜贴壁,DNA与转染试剂比例1:3 (μg:μL),转染步骤参照PEI说明书进行,转染后30小时置于显微镜下检测。
3. 结果与分析
3.1. A549细胞总RNA的提取与Nudel基因的扩增
培养A549细胞,选取生长状态良好的A549细胞为材料,提取总RNA。RNA浓度测定表明,1号样品浓度为730 ng/μL,A260/280比值为1.98,2号样品浓度为695 ng/μL,A260/280比值为1.97,说明提取的RNA 纯度比较高。提取的RNA进行电泳检测,结果见图1(A)。可以看到清晰的三条带,分别是28 S,18 S和5.8 S,表明提取的RNA完整性良好,可以用于反转录。
以总RNA为模板,反转录获得cDNA,再以cDNA为模板,扩增目的基因 Nudel。扩增结果见图1(B),电泳条带单一,对比DNA Marker,扩增的目的条带大小在1000 bp附近,与NCBI数据库里提供的Nudel基因(1038 bp)大小一致,初步判断扩增产物是目标产物,也说明A549细胞中Nudel基因有较高的表达水平。
3.2. 构建pT-Nudel和pDsRed-Nudel载体
将Nudel基因插入pMD18-T载体中,转化大肠杆菌DH5α,通过菌落PCR筛选阳性克隆,菌落PCR扩增结果见图2(A),在对应Maker1000 bp的位置有条带出现,与cDNA做模板扩增的条带大小一致。
 (A)
(A) 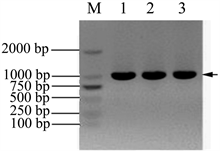 (B)
(B)
Figure 1. Amplification Nudel gene by RT-PCR. (A) The total RNA was extracted from A549 cells: 1 & 2 samples were the total RNA extracted from A549 cells; (B) Amplification of Nudel gene from the cDNA: M: DNA marker; 1, 2 & 3 samples were Nudel gene fragments amplified from the cDNA (about 1000 bp)
图1. RT-PCR扩增Nudel基因。(A) A549细胞总RNA提取电泳检测:1 & 2样品均为提取的A549细胞总RNA;(B)从A549细胞cDNA中扩增Nudel基因:M:DNA marker;1、2、3均为扩增的Nudel基因片段
 (A)
(A) 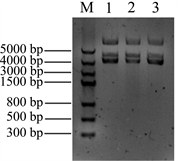 (B)
(B)
Figure 2. Construction the recombinants of pT-Nudel. (A) Identification the recombinant pT-Nudel by cloning PCR: M: DNA marker, 1, 2 & 3samples were the cloning PCR of recombinant pT-Nudel; (B) Isolation the recombinant plasmid pT-Nudel: M: DNA marker, 1, 2 & 3 samples were the recombinant pT-Nudel plasmids
图2. 构建重组质粒pT-Nudel。(A) 重组质粒pT-Nudel 菌落PCR鉴定:M:DNA marker,1、2&3为pT-Nudel菌落PCR;(B) 提取pT-Nudel重组质粒DNA:M:DNA marker,1、2&3为提取的重组质粒pT-Nudel
提取重组质粒DNA,电泳检测重组质粒大小,结果见图2(B),提取的质粒呈现3条带,中间的条带为质粒的线性条带,对比Marker,该线性条带在3000 bp和5000 bp之间,pMD18-T质粒大小为2692 bp,Nudel基因大小为1038 bp,重组质粒理论大小为3730 bp,从电泳结果判断,所提取的重组质粒大小正确,将正确的克隆进一步进行测序定。将测序结果在NCBI中用BLAST进行比对分析,本实验从A549肺泡细胞中所克隆的Nudel基因序列与NCBI数据库中的Nudel基因匹配度为100%,序列完整,并且没有发生碱基突变。
在此基础上,进一步将Nudel基因亚克隆至携带红荧光的真核表达载体pDsRed-C1上,亚克隆的限制性酶切位点为Xho I和BamH I,构建重组质粒pDsRed-Nudel。提取重组质粒,电泳检测见图3(A),pDsRed-C1载体大小为4700 bp,Nudel基因大小1038 bp,那么重组质粒pDsRed-Nudel的大小为5738 bp,由图3(A)中性条带大小判断,所提取的重组质粒大小正确。将提取的重组质粒pDsRed-Nudel用Xho I和BamH I进行双酶切鉴定,电泳结果显示(见图3(B)),在1000 bp和5000 bp的位置分别有条带出现,与预期相符,1000 bp附近的是Nudel基因片段,5000 bp附近的是载体片段。由此可见,Nudel基因成功的亚克隆到了红荧光表达载体上,构建了pDsRed-Nudel载体。
3.3. 重组质粒pDsRed-Nudel转染COS7细胞
利用PEI转染试剂,将重组质粒pDsRed-Nudel转染COS7细胞,检测其表达情况。结果如下(见图4),在平行转染条件下,pDsRed-C1空载体转染COS7细胞(见图4(B)),转染效率较重组质粒pDsRed-Nudel要高(见图4(D))。重组质粒pDsRed-Nudel转染COS7细胞的效率虽然较低,但是仍然观测到了荧光,初步判断pDsRed-Nudel在COS7细胞中能够表达。
 (A)
(A) 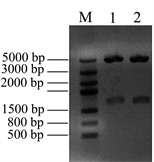 (B)
(B)
Figure 3. Construction the eukaryotic expression vector pRed-Nudel. (A) Isolation the recombinant pRed-Nudel plasmids: M: DNA marker; 1, 2, 3 & 4 samples were the recombinant pRed-Nudel plasmids; (B) Digestion the recombinant pRed-Nudel by restriction enzyme Xho I and BamH I: M: DNA marker; 1 & 2 samples were the recombinant pRed-Nudel digested by Xho I and BamH I
图3. 构建真核表达载体pRed-Nudel。(A) 提取重组质粒pRed-Nudel:M:DNA marker;1、2、3&4为提取的pRed-Nudel重组质粒;(B) 双酶切鉴定重组质粒pRed-Nudel:M:DNA marker;1&2为重组质粒pRed-Nudel双酶切
3.4. 抑癌基因Pten及其突变体对Nudel基因表达的影响
本实验克隆Nudel的目的,是为了考察Nudel对肿瘤细胞迁移的影响,而Pten基因是著名的肿瘤抑制基因,因此本实验将野生型的Pten和突变型的Pten即C124SPten (Pten第124位的氨基酸C突变成S),分别与Red-Nudel共转染COS7细胞,并在荧光显微镜下进行观察。结果发现,与突变型的C124SPten相比(见图5(D)),野生型的Pten与Red-Nudel共转染(见图5(B)),红荧光更少,推测124位的突变对Nudel的表达存在一定的影响。
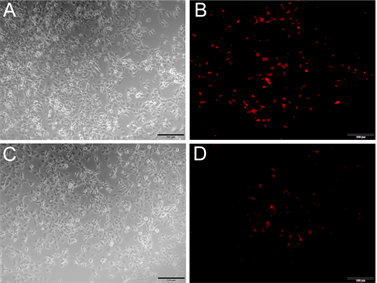
Figure 4. Transfection the expression vector pRed-Nudel into COS7 cells. (A) (B) Transfection the vector pDsRed-C1 into COS
7
cells, (A) At common optical path; (B) At fluorescence optical path; (C) (D) Transfection the expression vector pRed-Nudel into COS7 cells, (C) At common optical path; (D) At fluorescence optical path; Note: bar = 100 μm
图4. 重组质粒pRed-Nudel转染COS7细胞。(A) (B) pDsRed-C1转染COS7细胞,(A)图为白光,(B)图为激发光;(C) (D) pRed-Nudel转染COS7细胞,(C)图为白光,(D)图为激发光;注:图中比例尺为100 μm
4. 讨论
本实验以A549细胞为材料,成功获得目的基因Nudel,并构建携带红荧光报告基因的真核表达载体 pRed-Nudel。查阅Gene card可知,Nudel基因在骨髓普遍高表达,RPKM值为18.6,其次是肾上腺,RPKM
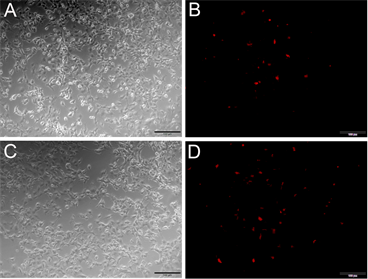
Figure 5. Co-transfection Pten or PtenC124S and pRed-Nude into COS7 cells. (A) (B) Co-transfection Pten and pRed-Nudel into COS7 cells, (A) At common optical path; (B) At fluorescence optical path; (C) (D) Co-transfection PtenC124S and pRed-Nudel into COS7 cells, (C) At common optical path; (D) At fluorescence optical path; Note: bar = 100 μm
图5. Pten和PtenC124S与pRed-Nudel共转染COS7细胞。(A) (B) Pten和pRed-Nudel共转染COS7细胞,(A)图为白光,(B)图为激发光;(C) (D) PtenC124S和pRed-Nudel共转染COS7细胞,(C)图为白光,(D)图为激发光;注:图中比例尺为100 μm
值为13.4。在另外的25种组织中均不同程度检测到Nudel的表达,具体表达情况见图6,在肺组织中的RPKM值约为7.97左右,RPKM值的大小显示的是该基因在相应的组织中表达量的高低。本实验所用的是A549细胞,该细胞为人肺泡上皮细胞。实验结果表明,Nudel基因在A549细胞中有表达,因此成功扩增到了该基因,测序结果显示,该基因在A549细胞株中,没有突变。

Figure 6. The Nudel gene expression level in different tissue
图6. Nudel基因在不同组织中的表达水平
Nudel是一种保守的中心体蛋白质,在细胞中绝大部分分布于细胞质,但在间期在中心体上有较强的定位,在有丝分裂期分布于整个纺锤体 [14] 。在体内,Nudel对于细胞运动的关键蛋白—胞质动力蛋白发挥功能至关重要,包括细胞内运输、有丝分裂和细胞迁移等。Nudel通过与Cdc42竞争结合Cdc42GAP,使得Cdc42释放而被活化,进而促进细胞迁移 [9] 。本实验初步尝试将野生型Pten和突变型PtenC124S与Red-Nudel共转染,观察到与突变型PtenC124S相比,野生型Pten对Red-Nudel的表达有抑制作用,需要进一步实验证实,如果Pten下调Nudel的表达,Nudel水平降低,则Nudel竞争结合Cdc42GAP水平降低,那么Cdc42GAP与Cdc42结合增加,则细胞迁移降低。也就是说,Pten通过下调Nudel进而抑制细胞迁移,这与Pten抑癌基因的功能是相符的 [15] 。
致谢
感谢湖北省自然科学基金资助项目(2015CFB490)的资助;感谢全国大学生创新创业大赛项目(201710488013)的资助。
NOTES
*通讯作者。