摘要:
目的:通过体外模拟实验对食管胃吻合口的生物力学进行分析并应用奈维补片加强吻合口局部的抗拉力。方法:选取食管癌术后复查胃镜的患者,通过计算分别得出颈部吻合口和胸部吻合口到食管入口的距离;通过在X线下测量头部活动时梨状窝下极的活动度来估测食管入口的位移;以上述测量结果为依据,应用新鲜猪食管、胃标本进行体外模拟实验,测量不同长度食管在相同位移下所受到的牵拉力,然后应用奈维补片加强食管胃吻合口,并测量吻合口所能承受的最大拉力。结果:1) 食管入口到颈部吻合口、主动脉弓上吻合口的平均距离分别为5.41 cm ± 1.22 cm、7.60 cm ± 1.36 cm;两者比较差异有统计学意义P = 0.000。2) 抬头和低头活动时,梨状窝下极平均活动范围为(2.26 ± 0.58) cm。3) 随着食管长度的增加,食管拉伸2 cm时所承受的拉力逐渐减小。并且食管长度每增加1cm,各组食管所承受的拉力值差异均有统计学意义P < 0.05。4) 常规吻合后吻合口承受最大拉力平均值为(20.00 ± 1.65) N,应用奈维加强后吻合口承受最大拉力平均值为(27.67 ± 0.95) N,两组比较差异有统计学意义(P = 0.001)。结论:颈部活动导致的食管入口位移会造成食管癌术后吻合口张力的增加,可能是导致吻合口瘘的重要因素;应用奈维补片可以增加吻合口的局部抗拉强度。
Abstract:
Objective: To analyze the biomechanics of esophagogastric anastomosis by in vitro simulation experiment and to strengthen the local tensile resistance of anastomosis by Neoveil®. Methods: Patients who underwent gastroscopy after esophageal cancer surgery were selected, and the distances from neck anastomosis and chest anastomosis to esophageal entrance were calculated respectively. Displacement of the esophageal inlet was estimated by X-ray measurement of the motion range of the lower pole of piriform recess during head movement. Based on the above measurement results, fresh pig esophagus and stomach samples were used for in vitro simulation experiment to measure the tensile force of different lengths of esophagus under the same displacement, Then Neoveil® was used to strengthen the esophagogastric anastomosis and the maximum tensile force of the anastomosis was measured. Results: 1) The average distance from the esophageal entrance to the neck anastomosis and the anastomosis above the aortic arch was 5.41 cm ± 1.22 cm and 7.60 cm ± 1.36 cm, respectively. The difference between the two was statistically significant P = 0.000. 2) The average motion range of the lower pole of piriform recess was (2.26 ± 0.58) cm when the head was raised and lowered. 3) As the length of the esophagus increases, the pulling force on the esophagus when stretched 2 cm decreases gradually. Moreover, for each 1 cm increase in esophageal length, the difference in esophageal tensile force between each group was statistically significant (P < 0.05). 4) After conventional anastomosis, the mean value of the maximum tensile force of the anastomosis was (20.00 ± 1.65) N, and after Neoveil® was used to strengthen the anastomosis, the mean value of the maximum tensile force of the anastomosis was (27.67 ± 0.95) N, the difference between the two groups was statistically significant (P = 0.001). Conclusion: The esophageal entrance displacement caused by neck movement will increase the anastomotic tension after esophageal cancer surgery, which may be an important factor leading to anastomotic fistula. The local tensile strength of anastomosis can be increased by using Neoveil®.
1. 引言
食管癌是消化道常见的肿瘤之一,据2018年统计结果显示,其发病率占恶性肿瘤发病率的第7位 [1]。在我国,其发病率和死亡率分别位居恶性肿瘤的第3位和第4位 [2] [3]。目前食管癌的治疗仍是以手术切除为主,而术后吻合口瘘是食管癌切除术后常见的危重并发症之一,随着食管癌手术标准化操作的普及和吻合器的应用,吻合口瘘发生明显下降,但据报道其发生率仍在5%~20%,且颈部吻合口瘘发生率明显高于胸内吻合 [4] [5] [6] [7] [8]。研究证明,吻合口的局部血运和吻合口所承受的局部张力是影响吻合口愈合的重要因素 [9] [10] [11],但是针对这些原因所做的技术改进并未完全控制吻合口瘘的发生。且食管癌切除术吻合部位与其所承受的张力之间的关系目前尚无报道。曾有研究显示,梨状窝下极与食管入口位置相对恒定,而抬头和低头动作会造成梨状窝下极的明显移位 [12],这就意味着食管入口部位也有着相同的位移,而这一位移可能会沿食管纵轴传导至吻合口,造成对吻合口的牵拉,从而影响吻合口的愈合。从食管切除术后吻合口的解剖特点发现,颈部吻合口到食管入口的距离小于胸内吻合口到食管入口的距离,因而颈部活动造成的牵拉力可能对颈部吻合口造成的影响亦较大。所以本研究拟通过对梨状窝下极活动度即食管入口活动度的测量来模拟食管癌术后吻合口所承受的纵向牵拉力,并根据实验结果局部应用奈维补片加强吻合口的抗拉力来预防吻合口瘘的发生。
2. 资料与方法
2.1. 食管入口至吻合口的距离测定
回顾性分析我院内镜科2018年1月至2018年10月接受食管癌根治术后复查行食管镜检查的患者207例。其中男性129例,女性78例;年龄39~76岁,平均(56.13 ± 5.81)岁;行腔镜下食管癌根治术150例,开式手术57例;其中弓上吻合140例,颈部吻合67例。纳入标准:1) 接受食管癌根治术,包括开式手术及腔镜下食管癌根治术;2) 经术后病理证实为食管癌;排除标准:1) 发生食管胃吻合口瘘;2) 局部复发;3) 全身状况差,不能耐受术后治疗及内镜复查。测量这些患者吻合口到门齿的距离,然后减去15 cm求得食管入口到吻合口的平均距离。
2.2. 梨状窝下极活动度测量
于我院放射科选取手术前行消化道造影的患者80例,其中男性59例,女性21例;年龄32~72岁,平均(50.29 ± 6.73)岁;中段食管病变59例,下段食管病变21例。嘱患者分别在抬头和低头状态下吞咽造影剂,测量梨状窝下极在抬头和低头状态时的位置,测量其位移距离。
2.3. 体外模拟食管抗拉力实验
选取新鲜猪食管上段由环装软骨下缘横断,向下测量自然长度为8 cm的食管予以横断。将食管上、下端固定于艾德堡数显测力计。将食管向下垂直快速拉长2 cm,由测力计读数观察最大值。以此类推,以同种方法分别测试长度为7 cm、6 cm、5 cm、4 cm、3 cm食管所受拉力,观察最大值。每个长度重复测量3次。操作均由同一组人员完成。
2.4. 吻合口最大抗拉力测定
选取新鲜猪食管和胃12套,平均分为常规吻合组和奈维加强组,每组6套,常规吻合组应用华森26号吻合器行食管–胃端侧吻合;奈维加强组吻合前将奈维补片覆于钉砧吻合面,再进行食管–胃端侧吻合,吻合后可见吻合口内夹衬一层奈维补片(如图1所示)。吻合后在距离吻合口约5.0厘米的两端分别将食管、胃封闭,并固定于艾德堡数显测力计的上下两端。胃腔内注入美兰,逐渐加大拉力器拉力,并密切观察是否吻合口有美兰漏出(如图2所示),由测力计读数观察最大值。

Figure 1. The tension resistance of anastomotic site was strengthened by the mesh (The arrow shows the effect of strengthening anastomosis)
图1. 应用补片加强吻合口抗拉力(箭头所示奈为加强吻合后效果)

Figure 2. The tension resistance of anastomotic site was measured after injection of methylene blue (The arrows shows the position of meilan injection and the moment of liquid leakage)
图2. 注入美兰后测量吻合口抗拉力(箭头所示为注入美兰位置及漏液瞬间)
2.5. 统计学分析
采用SPSS19.0统计软件进行统计分析,计数资料采用卡方检验,计量资料采用t检验。
3. 结果
3.1. 食管入口到吻合口距离
食管入口到颈部吻合口的平均距离为5.41 ± 1.22 cm,食管入口到主动脉弓上吻合口的平均距离为7.60 ± 1.36 cm;两者比较差异有统计学意义P = 0.000 (见图3)。

Figure 3. Comparison of distance between the entrance of esophagus and the anastomotic site
图3. 食管入口距吻合口距离的比较
3.2. 梨状窝下极活动度
抬头和低头状态下梨状窝下极平均活动范围为(2.26 ± 0.58) cm (测量方法如图4所示)。
1) 随着食管长度的增加,食管拉伸2 cm时所承受的拉力逐渐减小,每相差1 cm,各组食管所承受的拉力值差异均有统计学意义(见表1、图5)。
2) 常规吻合后吻合口承受最大拉力平均值为(20.00 ± 1.65) N,应用奈维加强后吻合口承受最大拉力平均值为(27.67 ± 0.95) N,两组比较差异有统计学意义(P = 0.001) (见表2)。
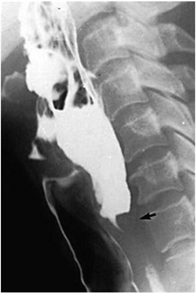
Figure 4. Displacement measurement of piriform recess (The lower pole of piriform recess is indicated by the arrow)
图4. 测量梨状隐窝位移(箭头所示为梨状隐窝下极)

Table 1. The tension of the pig esophagus in vitro when stretched by 2 cm (Unit: N)
表1. 离体猪食管拉伸2 cm时拉力测定(单位:N)
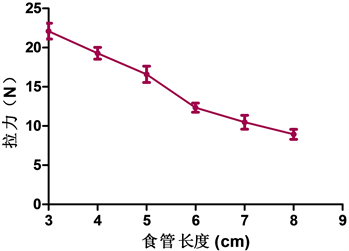
Figure 5. The tension of the esophagus of different lengths when stretched by 2 cm
图5. 不同长度的食管拉伸2 cm时所承受的拉力

Table 2. Comparison of maximum tension between conventional anastomosis group and reinforcement group (Unit: N)
表2. 常规吻合组及加强组所受最大拉力比较(单位:N)
3.3. 食管抗拉力测定
不同长度标本被拉伸2 cm时,承受的拉力值不相同,测量拉力的平均值,随着标本长度增加,承受拉力值逐渐降低。
3.4. 吻合口最大抗拉力测定
对常规吻合组标本以及应用奈维补片加强吻合口的标本进行了拉力测定,结果显示两组差异有统计学意义(p = 0.001),有奈维加强组的食管标本可承受更大的拉力。
4. 讨论
食管及胃肠道手术后吻合口局部血运及吻合口所承受的张力一直被认为是影响吻合口愈合的重要因素 [13] [14]。大量研究发现,食管癌术后颈部吻合口瘘的发生率明显高于胸内吻合口瘘 [15] [16]。我们通过对术后患者吻合口到食管入口距离的测量发现,颈部吻合口到食管入口的距离明显小于胸内吻合口到食管入口的距离,所以我们推测,吻合口到食管入口的距离越小即剩余食管长度越短,吻合口瘘的发生率越高;而颈部的活动造成的食管上部的位移可能是造成颈部吻合口局部张力增加的重要原因。
已有学者证实,食管入口与梨状窝下极的位置相对固定,可以用梨状窝的位移来代表食管入口的位移 [12]。我们分别在低头和抬头的情况下,对患者梨状窝的位移进行了测量,平均位移为2.26 ± 0.58 cm,与文献报道结果接近。所以,在后续的研究中,我们在体外进行了实验验证,将食管一端的位移距离设定为2 cm,发现,食管长度越短,食管所受的张力越大,随着食管长度的延长,其所受的拉力逐渐减小;所以,在颈部活动时,颈部吻合口所受的张力是明显大于胸内吻合口的,这可能在力学层面对颈部吻合口瘘发生率较高做出了解释。
奈维补片(Neoveil®)目前已广泛应用于临床,并有多项实验证实可以用来加强局部张力 [17] [18] [19],为了增加吻合口的抗拉力,我们在体外新鲜猪组织标本上采用了奈维补片加强吻合口的办法,并对吻合口进行了最大拉力的测量,发现,无奈维加强的情况下,吻合口所能承受的最大拉力与剩余食管长度为4 cm时食管位移2 cm所承受的拉力接近,甚至小于剩余食管长度为3 cm时食管位移2 cm所承受的拉力,这就说明,剩余食管小于4 cm时,抬头动作完全可能撕破吻合口,造成吻合口瘘,而应用奈维加强后,吻合部位抗拉力明显增加,且两组差异有统计学意义,这与其他研究结果一致。说明应用奈维加强后的吻合口抗拉力明显增强,这对于预防局部张力造成的吻合口瘘会有一定的预防作用;并且,限制食管癌术后患者的颈部活动可能有助于预防吻合口瘘的发生。
总之,通过本次实验,我们发现颈部活动会造成食管入口的位移,从而增加吻合口的局部张力,而且残留食管长度越短,吻合口的局部张力可能越大,这可能是造成颈部吻合口瘘发生率较高的重要原因;颈部制动或加强吻合口局部的抗拉能力可能会降低颈部吻合口瘘的发生,但需在临床治疗中进一步证实。
基金项目
此课题为2016年河北省政府资助省级临床医学优秀人才项目。
NOTES
*通讯作者。