摘要: 以石墨为原料电化学阳极剥离石墨烯引起了越来越多的关注,但在电化学剥离中离子嵌入非常快,石墨电极往往会瓦解,从而导致石墨烯产率较低且层数分布不均,电化学阴阳极交换剥离技术可有效改善石墨烯的质量。本实验以石墨箔作为对电极,基于电极交换剥离技术制备少层石墨烯。利用扫描电镜、透射电镜、原子力显微镜、拉曼光谱等表征,对比阴阳极交换前后两次剥离石墨烯的表面形貌、层数以及缺陷程度,并通过在DFM溶液中静置保存研究其稳定性。结果表明:相比于第一次剥离的石墨烯,第二次交换阴阳极后剥离得到的石墨烯边缘褶皱更明显,表现得更加透明,层数为单层或双层,且缺陷更小。通过在DMF溶液中静置保存,发现第一次剥离的石墨烯2 h就出现分层现象,而第二次阴阳极交换剥离的石墨烯保存220天不发生沉降,且随着交换次数的增加,石墨烯的稳定性越好。
Abstract:
Electrochemical anodic exfoliated graphene from graphite as a raw has attracted more and more attention. However, in the process of electrochemical stripping, ion embedding is very fast, and the graphite electrode tends to collapse, resulting in low graphene yield and an uneven layer dis-tribution. Electrochemical cathode and anode exchange stripping technology can effectively im-prove the quality of graphene. In this work, graphite foil was used as the counter electrode, and few layers of graphene were prepared based on electrode exchange stripping technology. Scanning electron microscopy, transmission electron microscopy, atomic force microscopy and Raman spectroscopy were used to characterize the surface morphology, the number of layers and defect degree of graphene peeled before and after anode exchange. And the stability of graphene was studied by keeping in DFM solution. The results showed that compared with the graphene peeled for the first time, the graphene obtained after the second exchange of anode and cathode peeled off had more obvious edge folds, more transparent, fewer defects, and the number of layers was single or double. By keeping in DMF, it was found that the exfoliated graphene for the first time showed delamination in 2 h, while the exfoliated graphene by the second cathode and anode exchange was stored for 220 days without delamination, and the stability of the graphene became better with the increase of exchange times.
1. 引言
石墨烯 [1] (Graphene, Gr)是继零维富勒烯 [2] (Fullerene)和一维碳纳米管 [3] (Carbon Nanotubes, CNTs)之后被发现的二维碳纳米材料 [4],如图1所示。石墨烯是由6个碳原子通过sp2结合组成,每个碳原子通过σ键与相邻的三个碳原子相连,碳碳键之间结构相同,原子间距为1.42 Å,键能较大,因此结构比较稳定。这种独特的结构使石墨烯具有极高的导电导热性能(室温下载流子的迁移率为15,000 cm2/(V∙s) [5],导热率为5000 W/(m∙K) [6],临界电流密度为108 A/cm2以及超高的力学性能(无缺陷的单层石墨烯杨氏模量约为1 TPa [7],断裂强度为130 GPa [7] ))。石墨烯这些非凡独特的特性,使其能够应用于纳米电子学 [8]、复合材料 [9] [10] [11] [12]、传感器 [13] 和下一代可再生能源技术(如太阳能电池 [14] )等多个领域。
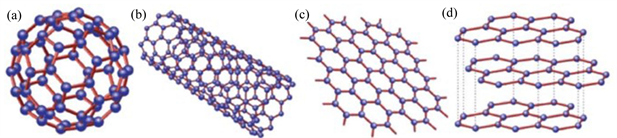
Figure 1. The isomer of carbon (a) fullerene (b) carbon nanotube (c) graphene (d) graphite
图1. 碳的同素异构体(a) 富勒烯;(b) 碳纳米管;(c) 石墨烯;(d) 石墨
电化学剥离法 [15] [16] [17] [18] 是一种简单、可扩展的技术,通常采用石墨作为工作电极,石墨、铂丝(Pt)等不同材料作为对电极,在电场的作用下,电解液发生氧化或还原反应并伴随着气体产生,电解液离子和气体插入石墨层中,使石墨膨胀产生剥离效果。相比于机械剥离,电化学剥离法所用的时间短,剥离几分钟就能达到大于70%且横向尺寸为1~10 um的单层或者双层石墨烯,可实现大规模生产。相比于液相剥离,电化学法制备石墨烯装置简单、环境友好,且制备的石墨烯含氧官能团(C=O,-COO-)少,碳结构完整,且可精准调节电流和电压实现操作的重复性和可控性,是制备石墨烯的有效方法。但直接用石墨箔或石墨棒作为工作电极时,随着离子插层的进行,所有石墨的边缘处剥离几乎同时发生,石墨电极往往会瓦解,这导致大多数膨胀石墨块体离开石墨阳极时还没有完全完成插层,掉落下来的石墨也将不再参与插层,从而导致石墨烯产率较低且质量分布不均匀。石墨箔的交换剥离可有效改善这一问题,石墨箔的交换剥离不仅可以改善石墨烯质量的不均匀性还可以多次利用有益于环保和节约成本。因此,本工作采用电化学阴阳极交换剥离技术来获得石墨烯,通过扫描电镜、透射电镜和原子力显微镜观察石墨烯的形貌和层数,并利用拉曼光谱表征石墨烯的缺陷,最后研究了石墨烯在DMF溶液中的稳定性。
2. 实验部分
2.1. 实验材料及设备
本实验使用石墨箔作为对电极剥离石墨烯,实验试剂为硫酸铵((NH4)2SO4),二甲基甲酰胺(DMF),均为分析纯。使用吉时利型号为(2260B-250-4)的电流源给电化学剥离石墨烯提供电流,使用冷冻干燥机冷冻干燥石墨烯。
2.2. 剥离石墨烯的机理
电化学剥离法剥离石墨烯主要经历插层、膨胀和剥落这三个过程。在外加电压的作用下,电解液解离出的阴离子向阳极移动,进而插入石墨层间,降低了石墨层间的范德华力。电解过程中水和阴离子分解产生气体促进石墨膨胀、剥离成片状单层或少层石墨烯。本实验的剥离机理如图2所示 [19],首先,施加电压会使阴极的水产生OH−,OH−在电解质中作为亲核试剂,主要剥离石墨的边缘位置和晶界。然后,石墨边缘开始膨胀,有利于
嵌入到石墨层中,硫酸根离子与水产生氧化还原反应,产生SO2和O2,最后,这些气体会插入到石墨层中使石墨层彼此分开。
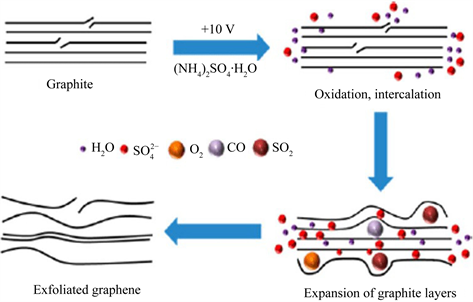
Figure 2. The diagram of electrochemical stripping mechanism
图2. 电化学剥离机理图
2.3. 石墨烯的制备方法
电化学阴阳极交换剥离流程如图3所示。配制硫酸铵电解液加热到40℃,将两片尺寸为3 cm × 5 cm × 0.1 cm的石墨箔分别放入镀槽中,一片石墨箔连接电源的正极作为电解阳极,另一片石墨箔连接电源的负极作为电解阴极。石墨箔间距恒定为2 cm,电压大小恒定为10 V,剥离时间为15 min。第一次剥离完后再配置相同浓度的电解液,然后交换阴阳极的石墨箔参照第一次剥离的实验参数再剥离15 min。最后将两次剥离得到的石墨烯分别在50 ml DMF溶液中超声分散2 h,洗涤过滤后冷冻干燥得到交换前后的石墨烯。

Figure 3. The Schematic diagram of electrochemical cathode and anode exchange exfoliation process
图3. 电化学阴阳极交换剥离流程示意图
2.4. 测试表征
石墨烯表面形貌测试采用场发射扫描电子显微镜FEI Inspect F50 (FSEM)。石墨烯结构与形貌测试采用高分辨透射电子显微镜FEI Tecnai G2 F20 (TEM)。石墨烯层数表征采用原子力显微镜Bruker dimension icon (AFM),石墨烯缺陷程度测试采用拉曼分析仪HORIBA FRANCE SAS (Raman)。
3. 结果与讨论
3.1. 石墨烯的表面形貌
扫描电镜经常被用来表征电化学剥离法制备的石墨烯,由于石墨烯覆盖的区域在电子束中带正电,所以石墨烯的形状和尺寸可以通过扫描电镜成像。图4为石墨烯的SEM表面形貌图。图4(a)、图4(b)为第一次剥离的石墨烯,图4(c)、图4(d)为第二次剥离的石墨烯。可以看出第一次剥离的石墨烯具有良好的片层状结构,边缘无褶皱,较厚层数多。经过第二次阴阳极交换剥离石墨烯后,石墨烯呈轻纱薄片状,边缘有明显的褶皱且更透明,具有石墨烯表面形貌的典型特征,正是因为这些褶皱的存在克服了石墨烯层之间的应力,石墨烯才能在自然界中以二维的形式稳定存在,也正是这些褶皱使石墨烯得以在显微镜下进行形貌观察。
3.2. 石墨烯的层数表征
由于石墨烯具有较高的表面能,在自由状态下石墨烯自发的发生扭曲以获得热力学稳定状态,因此在TEM图中能看到褶皱现象,这种现象同时也说明了石墨烯的片层极薄。为了判断石墨烯的层数,分别对第一次和第二次剥离的石墨烯进行了TEM和AFM表征。第一次和第二次剥离石墨烯的TEM立体结构如图5所示,其中图5(a)~(d)为第一次剥离的石墨烯,图5(e)~(h)为第二次剥离的石墨烯。从图5(a)、图5(b)、图5(e)、图5(f)可以看出,两次剥离的石墨烯都呈片层状结构,但第二次剥离的石墨烯边缘更加透明,褶皱更明显,说明第二次剥离的石墨烯质量更好。高分辨率电镜可以从片层翘起的边缘和突起台阶的个数,估计片层石墨烯的层数。从图5(c)、图5(d)、图5(g)、图5(h)可以判断出,第一次剥离的石墨烯为多层结构,为5到6层,第二次剥离的石墨烯呈现单层或双层结构。
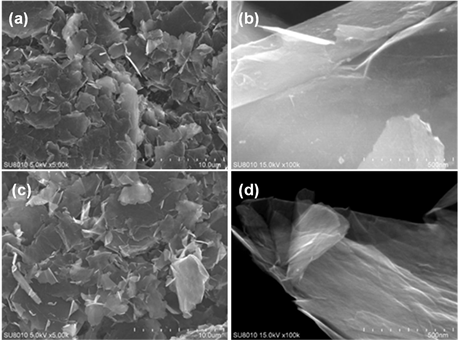
Figure 4. SEM images of exfoliated graphene for the first time: (a) SEM image of low magnification; (b) SEM image of high magnification; SEM images of exfoliated graphene for the second cathode and anode exchange: (c) SEM image of low magnification; (d) SEM image of high magnification
图4. 第一次剥离石墨烯后的SEM图:(a) 低倍SEM;(b) 高倍SEM;第二次阴阳极交换剥离石墨烯后的SEM图:(c) 低倍SEM;(d) 高倍SEM

Figure 5. TEM images of exfoliated graphene for the first time: (a) TEM image of low magnification; (b) TEM image of high magnification; (c), (d) Edges and raised steps of grapheme; TEM images of exfoliated graphene for the second cathode and anode exchange: (e) TEM image of low magnification; (f) TEM image of high magnification; (g), (h) Edges and raised steps of graphene
图5. 第一次剥离石墨烯的TEM图:(a) 低倍TEM立体结构;(b) 高倍TEM立体结构;(c)、(d)石墨烯的边缘和突起台阶;第二次剥离石墨烯的TEM图:(e) 低倍TEM立体结构;(f) 高倍TEM立体结构;(g)、(h) 石墨烯的边缘和突起台阶
为了对石墨烯的厚度和层数进行更精准的表征,利用AFM对衬底上的石墨烯片层进行全局扫描,扫描区域为5 μm2。如图6所示,图6(a)、图6(b)为第一次剥离石墨烯的AFM图,图6(b)为图6(a)中白色线对应的高度轮廓图。图6(c)、图6(d)为第二次剥离石墨烯的AFM图,图6(d)为图6(c)中白色线对应的高度轮廓图。理论上单层石墨烯的厚度为0.34 nm,从扫描曲线来看,第一次剥离的石墨烯横向尺寸约为0.5~1 um,平均厚度约为2 nm,其相当于5~6层的石墨烯;第二次剥离的石墨烯横向尺寸约为0.5~1 um,平均厚度约为1 nm,相当于2~3层的石墨烯,这与TEM表征结果一致。

Figure 6. AFM images of exfoliated graphene for the first time: (a) Two-dimensional topography of AFM; (b) Thickness measurement. AFM images of exfoliated graphene for the second cathode and anode exchange: (c) Two-dimensional topography of AFM; (d) Thickness measurement
图6. 第一次剥离石墨烯的AFM图:(a) AFM二维形貌图;(b) 厚度测量图;第二次阴阳极交换剥离石墨烯的AFM图:(c) AFM二维形貌图;(d) 厚度测量图
3.3. 石墨烯的拉曼光谱表征
利用拉曼光谱对石墨烯的缺陷进行表征,如图7所示。图中分别为第一次剥离石墨烯、第二次剥离石墨烯和原始石墨箔的拉曼光谱。可以看出石墨箔的2D峰为非对称峰,这是石墨拉曼光谱图的典型特征。两次剥离的石墨烯都有D峰,G峰及2D峰,这是石墨烯拉曼图的典型特征峰。D峰和G峰的峰强比可以反应石墨烯的缺陷程度,其比值越小,缺陷越小,石墨烯质量越好。第一次剥离的石墨烯ID/IG = 0.29,第二次剥离的石墨烯ID/IG = 0.10,表明第二次剥离的石墨烯晶格缺陷小、质量高。拉曼光谱中2D峰与G峰的峰强比还可以反应石墨烯的层数,其比值越大层数越少,石墨烯质量越好。第一次剥离的石墨烯I2D/IG = 0.24,第二次剥离的石墨烯I2D/IG = 0.74,表明第二次剥离的石墨烯层数更少,质量更好。一般认为,I2D/IG < 0.7为多层石墨烯,I2D/IG约为0.7~1.4为双层石墨烯,I2D/IG > 1.4为单层石墨烯。所以第一次剥离的石墨烯为多层石墨烯,第二次剥离的石墨烯为少层石墨烯,这与前面的TEM和AFM表征结果一致,且第二次剥离的石墨烯缺陷小质量高。

Figure 7. Raman spectrum of exfoliated graphene for the first time and exfoliated graphene for the second cathode and anode exchange
图7. 第一次剥离石墨烯和第二次阴阳极交换剥离石墨烯的拉曼光谱
3.4. 石墨烯在DMF溶液中的稳定分散
由于DMF的表面张力为37 mN/m,与石墨烯的表面张力接近,分散助剂吸附在石墨烯表面,能起到稳定分散石墨烯的作用。因此,在超声下既可以剥离石墨烯,同时也可以考察石墨烯在溶剂中的稳定性。石墨烯在DMF溶液中没有出现分层现象,表明石墨烯在DMF溶液中分散良好,即稳定性越好。如图8所示,图8(a)~(d)分别为静置2 h、220天、300天、350天后石墨烯的分散情况。图中编号(1)为第一次剥离的石墨烯,编号(2)为第一次交换阴阳极后剥离的石墨烯,编号(3)为第二次交换阴阳极后剥离的石

Figure 8. Dispersion stability of exfoliated graphene for the first time and exfoliated graphene of multiple anode and cathode exchange in DFM solution. (a) 2 hours; (b) 220 days; (c) 300 days; (d) 350 days
图8. 第一次剥离石墨烯和多次阴阳极交换剥离石墨烯在DFM溶液中的分散稳定性。(a) 2 h;(b) 220天;(c) 300天;(d) 350天
墨烯,编号(4)为第三次交换阴阳极后剥离的石墨烯。图8(a)中可以看出编号(1)溶液在静置2h后上层液体变澄清,出现了分层现象,编号(2)、(3)、(4)溶液未出现分层现象。从图8(b)~(d)中可以看出当保存时间为220天时,编号(2)、(3)、(4)溶液仍然未出现分层现象,当保存时间为300天时,编号(2)溶液出现了分层现象,编号(3)、(4)溶液依然未出现分层现象,当保存时间为350天时,所有溶液出现分层现象。这表明交换阴阳极后剥离的石墨烯在DMF中分散性好且稳定,第一次交换阴阳极后剥离的石墨烯可至少保存220天,且随着交换次数的增加石墨烯在DMF溶液中的稳定性越好。
4. 结论
利用电化学阴阳极交换剥离技术制备石墨烯,对比第一次剥离石墨烯和第二次阴阳极交换剥离石墨烯的表面形貌、立体结构、层数和缺陷,研究了第一次和第二次剥离的石墨烯在DFM溶液中的稳定性,由研究结果得到如下结论:
1) 第二次阴阳极交换剥离的石墨烯边缘有明显的褶皱,呈轻纱状,具有石墨烯形貌的典型特征。
2) 第二次阴阳极交换剥离的石墨烯相较于第一次剥离的石墨烯层数更少,缺陷更小,质量更高。
3) 第二次阴阳极交换剥离的石墨烯可在DMF溶液中稳定保存220天,且随着阴阳极交换次数的增加,石墨烯在DMF溶液中更加稳定。