摘要: 目的:观察药物涂层球囊(drug-coated balloon, DCB)与金属裸支架( bare metal stent, BMS)治疗下肢动脉硬化闭塞症(ASO)长段病变的疗效。方法:笔者通过选取2016年12月至2019年12月青岛大学附属医院血管外科接受介入治疗的老年下肢ASO患者,共73条患肢(病变长度 ≥ 20 cm)。每条患肢在选择合适的手术入路后使用普通球囊预扩张,然后选取符合靶病变的药物涂层球囊(DCB)或金属裸支架(BMS)植入,其中接受DCB治疗(包括单纯DCB和DCB + 短支架植入)的有36条患肢(DCB组),接受BMS治疗的有37条患肢(BMS组)。观察术后1个月、3个月、6个月、9个月、12个月血管通畅率及靶病变血管再重建率,期间无患者死亡、截肢。采用SPSS 22.0软件进行统计分析。根据数据类型,组间比较采用t检验或χ
2检验。结果:73条患肢均获得技术成功,且随访率为100%。观察2组患肢术后12个月血管通畅率及靶病变重建率。结果:DCB组的术后12月靶病变通畅率为86.1%,BMS组的通畅率86.4%,差异无统计学意义(P > 0.05),但是DCB组术后12月的临床驱动的靶病变重建率更低(DCB: 11.1% VS BMS: 48.6%),差异有统计学意义(P < 0.05)。结论:药物涂层球囊在治疗下肢动脉硬化闭塞症(病变长度 ≥ 20 cm)与金属裸支架在近期疗效上无显著差异,但相较于金属裸支架植入,药物涂层球囊有更少的术后管腔丢失,更低的血管再重建率。
Abstract:
Objective: To observe the efficacy of drug-coated balloon (DCB) and bare metal stent (BMS) in the treatment of long-segment lesions of lower extremity arteriosclerosis obliterans (ASO). Methods: The author selected 73 elderly patients with lower extremity ASO (lesion length ≥ 20 cm) who underwent interventional treatment in the Department of Vascular Surgery, Qingdao University Affiliated Hospital from December 2016 to December 2019. After selecting the appropriate surgical approach, each affected limb is predilated with a common balloon, and then a drug-coated balloon (DCB) or bare metal stent (BMS) that meets the target lesion is selected for implantation, and DCB treatment (including simple DCB and DCB + short stent implantation) had 36 affected limbs (DCB group), and 37 affected limbs (BMS group) received BMS treatment. The primary vascular patency rate and target lesion vascular remodeling rate were observed at 1 month, 3 months, 6 months, 9 months, and 12 months postoperatively. During this period, no patients died or had amputations. We use SPSS 22.0 software for statistical analysis. According to the data type, the t test or the χ2 test is used for comparison between groups. Results: All 73 affected limbs were technically successful, and the follow-up rate was 100%. The primary vascular patency rate and target lesion reconstruction rate were observed in the two groups at 12 months postoperatively. Results: In DCB group, the patency rate of target lesion was 86.1% 12 months after operation, and the patency rate of target lesion in the BMS group was 86.4%. The difference was not statistically significant (P > 0.05). However, the clinical driven target lesion reconstruction rate was lower in DCB group 12 months after surgery (DCB: 11.1% VS BMS: 48.6%), and the difference was statistically significant (P < 0.05). Conclusion: There is no significant difference between drug-coated balloons in the treatment of lower extremity arteriosclerosis obliterans (lesion length ≥ 20 cm) and bare metal stents in the short-term efficacy, but compared with bare metal stent implantation, drug-coated balloons have less. The postoperative lumen is lost, and the rate of vascular reconstruction is lower.
1. 引言
下肢动脉硬化闭塞症(arteriosclerosis obliterans, ASO或者peripheral arterial disease, PAD)是指各种因素引起的以下肢动脉(如股、腘、胫前、胫后动脉)狭窄甚至闭塞为主要表现的一类疾病,主要症状为:间歇性跛行、静息痛甚至下肢坏疽。随着经济社会发展与人口老龄化社会的到来,ASO逐渐成为威胁老年人身体健康的重要因素。下肢动脉硬化是全身动脉粥样硬化的一部分,类似于冠状动脉粥样硬化,目前认为病因为吸烟、高血压、糖尿病等因素导致下肢动脉管壁受损,脂质斑块沉积,或继发血栓形成,导致动脉管壁逐渐增厚,最终导致管腔狭窄、闭塞,引起患肢缺血性改变,如:肢体疼痛、发凉、坏疽,甚至截肢 [1] [2] [3]。年龄、吸烟、高血压、高血脂、糖尿病是导致ASO的独立危险因素 [4]。二十世纪以来,随着医疗水平和医疗器械的发展,血管腔内治疗下肢动脉硬化闭塞症取得了突飞猛进的发展,已成为传统外科手术之外的另外一种重要的治疗方式 [5] [6] [7]。目前已成为治疗下肢动脉硬化闭塞症(ASO)的主要治疗方式,血管腔内治疗相比于传统外科手术,明显降低了手术并发症、并缩短了手术时间,得到越来越多血管外科医生的青睐 [8]。传统的普通球囊扩张+金属裸支架置入(POBA + BMS),具有较高的成功率和可靠的短期通畅率,但随之而来的支架内再狭窄(instent-restenosis, ISR)却是一个不可回避的术后常见并发症,有研究表明:下肢动脉支架植入术后ISR 12、24个月发生率可达30%、50%,许多患者不得不再次行血管腔内治疗。因此,人们提出并发明了药物涂层球囊(drug-coated balloon, DCB):药物涂层球囊表面覆盖有抗血管壁增生药物(如雷帕霉素、紫杉醇及其衍生物等),通过球囊扩张,药物涂层球囊表面的抗增生药物有效持续的扩散,吸附到血管壁,通过稳定细胞内微管,抑制血管壁平滑肌细胞的有丝分裂,从而实现阻止内膜增生,有效降低管腔再狭窄的作用。另外,一些解剖条件不适合支架植入的病变(跨关节部位的病变),DCB也可以发挥其重要的作用,手术效果明显提高,降低了支架内再狭窄的发生率,在某些情况下避免了支架的植入,为以后的治疗提供了更多的可能性 [9]。DCB治疗下肢动脉硬化闭塞症方面,目前主要应用于血管短段狭窄或闭塞(病变长度 < 10 cm),较少应用于长段狭窄或者闭塞(病变长度 > 20 cm),长段病变目前仍以金属裸支架植入为主。因此,为了研究DCB在治疗ASO长段病变的近期疗效,选取青岛大学附属医院血管外科行DCB或BMS治疗的ASO长段病变的73例患者进行回顾性研究,报告如下。
2. 对象与方法
2.1. 研究对象
病例资料为我院血管外科2016年12月至2019年12月收治的73例老年ASO患者(均为单侧患肢)。【纳入标准:年龄为18~85岁ASO患者,且均有较严重的间歇性跛行或静息痛(Rutherford分级均为2~4级),所有患者患肢住院前均未行手术治疗,病变血管完全闭塞、或狭窄程度 ≥ 70%;术前下肢动脉CTA提示(病变长度 ≥ 20 cm),且能被一个或多个支架/球囊覆盖】。【排除标准:全身严重感染、凝血功能异常、严重肾衰、对造影剂过敏、妊娠或哺乳期妇女等有手术禁忌的患者、下肢动脉急性缺血或急性血栓形成需要急症手术的患者、本次手术前3月有ST段抬高型心肌梗塞、出血性脑卒中或者重大外科手术的患者】。根据手术当中情况决定组别,DCB组共选取36例患肢,BMS组共选取37例患肢。本次研究已通过青岛大学附属医院医学伦理委员会伦理审批。
2.2. 方法
所有患者术前行心脏超声、颈部血管超声、胸部CT、经颅多普勒等影像学检查及常规生化检验,确定患者无明显手术禁忌症,能耐受手术;术前/术后均测踝肱指数ABI。所有患者术前均予以抗血小板、降脂治疗。病人取平卧位,常规消毒铺巾后,穿刺同侧或对侧股动脉(局部浸润麻醉),穿刺成功后置入5F导管鞘,并进入导丝导管,全身肝素化(静脉推注60~80 U/kg普通肝素),膝上动脉选用0.035英寸导丝通过病变段,膝下动脉选用0.018英寸导丝通过病变段,造影确认病变部位、长度、范围以及流出道情况,先选用直径4~5 mm普通球囊预扩张(扩张时间2~3 min)。
1) DCB组:普通球囊预扩张后,若扩张后血管无明显狭窄/回缩、限流及夹层形成,则立即紫杉醇药物涂层球囊(脉科)扩张,保证DCB长度至少超过病变血管两端0.5 cm,扩张时间至少2 min,术后造影,股腘动脉段通畅,且保证至少一条膝下动脉流出道通畅,则定义手术成功。若DCB扩张后病变血管有局部狭窄或者夹层,则给予补救性支架植入(见图1,图2)。
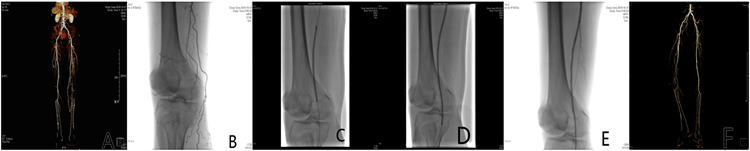 图A. 术前CTA:右侧股浅动脉长段闭塞;图B. DSA造影:右侧股浅动脉长段闭塞;图C. DSA造影:普通球囊扩张闭塞段;图D. DSA造影:DCB球囊扩张闭塞段;图E. DSA造影:DCB扩张后血流通畅;图F. 术后12月复查CTA。
图A. 术前CTA:右侧股浅动脉长段闭塞;图B. DSA造影:右侧股浅动脉长段闭塞;图C. DSA造影:普通球囊扩张闭塞段;图D. DSA造影:DCB球囊扩张闭塞段;图E. DSA造影:DCB扩张后血流通畅;图F. 术后12月复查CTA。
Figure 1. Treatment of ASO with DCB alone
图1. 单纯DCB治疗ASO
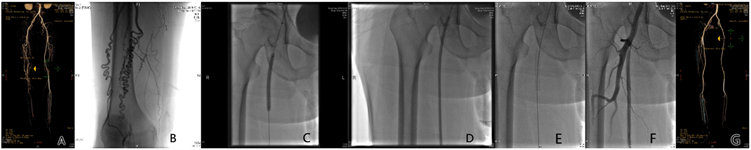 图A. 术前CTA:右侧股浅动脉长段闭塞;图B. DSA造影:右侧股浅动脉长段闭塞;图C. DSA造影:普通球囊扩张闭塞段;图D. DSA造影:DCB球囊扩张闭塞段;图E. DSA造影:DCB扩张后置入短支架;图F. DSA造影:置入短支架后血流通畅;图G. 术后12月复查CTA。
图A. 术前CTA:右侧股浅动脉长段闭塞;图B. DSA造影:右侧股浅动脉长段闭塞;图C. DSA造影:普通球囊扩张闭塞段;图D. DSA造影:DCB球囊扩张闭塞段;图E. DSA造影:DCB扩张后置入短支架;图F. DSA造影:置入短支架后血流通畅;图G. 术后12月复查CTA。
Figure 2. Treatment of ASO with DCB + short stent
图2. DCB + 短支架治疗ASO
2) BMS组:普通球囊预扩张后,若病变血管有较长狭窄限流或夹层,则植入金属裸支架,保证支架长度至少超过病变血管两端0.5 cm。术后造影,股腘动脉段通畅,且保证至少一条膝下动脉流出道通畅,则定义手术成功(见图3)。
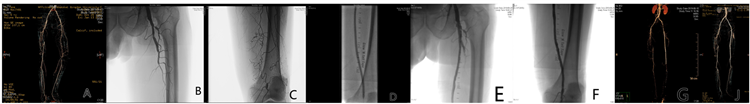 图A. 术前CTA:拟处理左侧股浅动脉长段闭塞;图B. DSA造影:左侧股浅动脉开口闭塞;图C. DSA造影:左侧股浅动脉长段闭塞;图D. DSA造影:普通球囊扩张闭塞段;图E/F. DSA造影:置入后置入短支架;图G/J. 术后5月/12月复查CTA。
图A. 术前CTA:拟处理左侧股浅动脉长段闭塞;图B. DSA造影:左侧股浅动脉开口闭塞;图C. DSA造影:左侧股浅动脉长段闭塞;图D. DSA造影:普通球囊扩张闭塞段;图E/F. DSA造影:置入后置入短支架;图G/J. 术后5月/12月复查CTA。
Figure 3. Treatment of ASO with BMS
图3. BMS治疗ASO
3) 共有73条患肢,其中36条患肢接受DCB治疗(DCB组,紫杉醇),37条患肢接受BMS治疗(BMS组)。所有患肢均为长段病变(病变长度 ≥ 20 cm)。手术完成后,均予以心电监护、穿刺点加压包扎24 h、经外周泵入普通肝素抗凝。出院后长期口服拜阿司匹林(100 mg Qn口服)及拜瑞妥(10 mg Qd口服)至少3个月,并嘱患者严格戒烟、控制血压、血糖等基础疾病,并注意患者功能锻炼。
2.3. 术后随访
对所有患者术后1月、3月、半年、9月、一年进行门诊随访。1) 复查下肢血管超声或下肢动脉CTA,判断血管通畅率。2) 记录患肢术前/术后ABI、截肢率、死亡率、记录术后12月靶病变再重建率,以判断血管通畅及血运情况。
2.4. 统计学处理
采用SPSS 22.0软件进行统计分析。计量资料用均数±标准差(x2 ± s)表示,组间比较采用t检验。计数资料用例数(百分率)表示,组间比较采用χ2检验,并绘制Kaplan-Meier曲线对术后通畅率及免于靶病变再重建率进行分析(见图4,图5)。P < 0.05为差异有统计学意义。
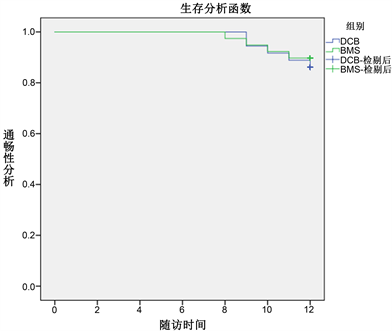
Figure 4. Kaplan Meier analysis of patency rate of target lesion in two groups at 12 months after operation
图4. 两组病人术后12月靶病变血管通畅率比较的Kaplan-Meier分析
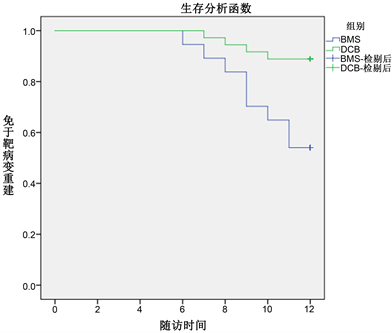
Figure 5. Kaplan Meier analysis of patients in two groups who were free of target lesion reconstruction 12 months after operation
图5. 两组病人术后12个月免于靶病变再重建的Kaplan-Meier分析
3. 结果
3.1. 2组患肢一般资料比较
2组患肢一般资料及病变血管特点方面比较,比较患者年龄、性别、吸烟、糖尿病、脑梗死等差异无统计学意义(P > 0.05;见表1)。比较患者冠心病等指标差异有统计学意义(P < 0.05;见表1)。

Table 1. Basic characteristics of two groups of data
表1. 两组资料的基本特征
3.2. 2组患肢手术效果比较
2组患肢手术成功率、随访率均为100%。随访期间没有任何手术或者设备相关的死亡病例,也未发生与手术或器械相关的出血、严重截肢等不良临床事件。术后12个月,2组患肢截肢率、术前/术后ABI差值、术后12月病变血管通畅率,差异无统计学意义(P > 0.05;见表2)。说明药物涂层球囊在治疗下肢动脉硬化闭塞症(病变长度 > 200 mm)与金属裸支架在近期疗效上无显著差异,但DCB组术后12月的临床驱动的靶病变重建率更低,差异有统计学意义(P < 0.05;见表2)。

Table 2. Clinical results of key data
表2. 关键数据的临床结果
4. 讨论
· 随着人预期寿命的延长,下肢动脉硬化闭塞症越来越普遍,已成为危害老年人生活质量与预期寿命的重要因素 [10]。随着人们生活水平与医疗器械的发展,越来越多治疗ASO的方式不断涌现:从最初的人工血管–搭桥手术到血管腔内技术再到新兴的生长因子注射治疗等。其中血管腔内技术血管因其具有创伤小、恢复快、手术时间短、手术风险低等优势,逐渐替代传统的外科手术,成为ASO主要的治疗手段 [11] [12] [13]。目前,血管腔内介入治疗ASO的主要方法有普通球囊扩张(POBA)、普通球囊扩张 + 金属裸支架置入(POBA + BMS),斑块旋切术、药物涂层球囊(DCB)等 [14]。其中,DCB和POBA + BMS治疗ASO最为广泛 [15]。近些年来,DCB技术发展迅速,且应用于冠状动脉及股腘动脉病变,因其表面涂有抑制血管壁平滑肌细胞增生的药物,从而达到抑制管壁内膜增生,减少术后病变血管再狭窄的发生 [16]。目前DCB国内应用最广泛的是紫杉醇涂层球囊,它通过稳定微管形成、减轻炎症反应,达到抑制血管壁平滑肌细胞增生,减少管腔再狭窄发生的风险 [17] [18] [19]。另外,DCB还可以治疗跨关节病变等一些不适合支架植入的部位,一定程度上避免了支架的植入,从而减少了支架内再狭窄和支架变形、断裂的风险,并且不影响后续的治疗,为以后的治疗提供了更多的可能性等 [20]。因此,DCB是作为一种可靠的治疗手段,在下肢动脉腔内治疗方面发挥越来越重要的作用 [21] [22] [23]。
· 本研究结果显示,虽然DCB治疗下肢动脉闭塞长段病变(病变长度大于20cm)在术后12月通畅率、踝肱指数等方面短期内无明显差异,但DCB可减低靶病变再重建率(DCB11.1%BMS48.6%),降低支架的使用率,提高患者的生活质量。
· 总之,DCB作为一种新兴的治疗手段,有广阔的发展前景。本研究可能存在以下不足:1) 病例资料较少;2) 随访时间较短;3) 有些患者依从性较差,未能严格戒烟,或者血压、血糖控制不理想;4) 患者术后复查未能行DSA检查。
NOTES
*通讯作者。