1. 前言
创伤性脑损伤(TBI),又称颅脑损伤,是由外伤所致的脑组织损伤,其不良预后已成为现代社会的重要问题 [1],且目前尚无有效治疗方法。炎症反应及严重的脑水肿是TBI后24 h急性期病理进展的关键因素 [2],其直接或间接导致的神经元损伤与多器官病理改变,常贯穿该疾病始终,最终导致不良预后。
细胞焦亡是近年新发现的一种细胞程序性死亡形式,其主要通过Caspase-1介导,引起细胞穿孔,最终导致炎症反应级联放大 [3]。细胞焦亡广泛参与多种疾病的发生、发展,其显著特征为IL-1β、IL-18向胞外大量释放 [4],最终引起全身炎症反应。
分子氢常以氢气的形式存在于自然界,而其作为一种新兴的医学气体,有易制得、低成本、起效快、无毒、无残留等特点,目前已被证实可通过抗炎、抗氧化应激、抗凋亡等途径改善多种器官损伤 [5] [6],但其具体作用机制尚未明确。目前研究显示,分子氢存在剂量依赖性,较高浓度的氢气可发挥更好的效果 [7]。因此,本实验通过液压冲击损伤(FPI)法建立TBI模型,探索较高浓度的分子氢对小鼠TBI后脑水肿及神经转归的影响,以及细胞焦亡机制与全身炎症反应的作用。
2. 材料和方法
2.1. 试剂及仪器
兔抗小鼠GAPDH (1:5000,SAB,#21612,美国),兔抗小鼠Caspase-1 (1:2000,Origin,#TA323204S,美国),兔抗小鼠ASC (1:2000,Origin,#TA326289S,美国);酶联免疫吸附测定(enzyme linked immunosorbent assay, ELISA)试剂盒(江莱生物,#IL-1β;#IL-18,中国);AMS-H-03氢氧气雾化机(上海潓美医疗科技,中国),液压冲击装置(AmScien Instruments,美国),尼氏染色液(索莱宝,#G1430,中国)。
2.2. 动物与分组
本实验经哈尔滨医科大学附属第二医院医学伦理委员会动物实验伦理审批(审批号:SYDWGZR2020-039)。选用雄性健康成年的无特定的病原体(specific pathogen free, SPF)级C57BL/6小鼠(10至12周龄),体重25~30 g [哈尔滨医科大学实验动物学部提供,许可证号:SCXK (黑) 2013-001],术前禁食6 h,不禁饮。所有实验动物使用随机数法分成3组:假手术组(S组)、创伤性脑损伤组(T组)和分子氢治疗组(H组),每组24只。
2.3. 模型制备
建立改良的小鼠颅脑液压冲击损伤(FPI)模型 [8],即TBI模型。腹腔注射戊巴比妥钠(35 mg/kg)麻醉小鼠,行气管插管,保留自主呼吸。术区常规消毒、备皮,1%利多卡因局部浸润麻醉,固定小鼠头部于脑立体定位仪(Narishige,SR-6N,日本),沿正中线切开,暴露颅骨,于矢状缝右侧1.2 mm、前囟门后1.2 mm处钻出直径约1 mm圆孔,保持硬脑膜完整无缺损。将液压损伤装置垂直于圆孔与小鼠硬脑膜形成紧密连接,确保完全密闭后,将颅脑液压损伤装置的摆锤调定于13˚,松开摆锤使其自由下落,液压装置中无菌生理盐水产生的压力(约1.5~2.0 atm)瞬时作用于硬脑膜,导致小鼠发生中度创伤性脑损伤。对于小鼠脑创伤后普遍发生的呼吸暂停现象,使用呼吸气囊通过气管插管辅助呼吸,术后对小鼠保温并监护至完全苏醒。
2.4. 分子氢治疗模式
采用潓美AMS-H-03氢氧气雾化机按1:2的比例电解去离子水 [9],使用氮气进行充盈,形成42% H2-21% O2-37% N2的混合气体。吸入过程在配有气体回路、呼出气吸收装置、气体浓度监测仪的防爆箱中进行。S组和T组全程吸入空气(21% O2-78% N2),H组在术后即日起每日吸入含42%氢气的混合气体2 h进行分子氢治疗。
2.5. 脑含水量测定
于损伤后48 h,戊巴比妥钠深麻醉下断头迅速分离脑组织,将损伤侧的大脑半球置于干燥培养皿中,使用电子天平称取重量,即为湿重;随后在80℃恒温烘干箱中干燥72 h,立即称取重量,即为干重。按照以下公式计算脑含水量:脑含水量 = (湿重 − 干重)/湿重 × 100%。
2.6. 脑组织病理观察
术后48 h,戊巴比妥钠深麻醉下经心尖穿刺,依次行生理盐水灌注和4%多聚甲醛固定,后断头取脑,置于4%多聚甲醛溶液中4℃固定24 h,常规石蜡包埋、冠状连续切片(3 μm),分别取脑组织切片进行苏木素-伊红(HE)染色和尼氏(Nissl)染色。低倍镜下(×40)取相同皮质形状切片,视为脑组织同一平面,于高倍镜下(×400)观察神经元细胞损伤和尼氏小体。
2.7. Western blot检测
术后24 h,深麻醉下快速断头取脑,取损伤半暗带皮质,RIPA裂解液裂解,4℃下14,000 r/min离心20 min吸取上清,BCA试剂盒测定蛋白浓度。使用10%的SDS-PAGE胶对蛋白样品进行电泳分离,上样,电泳,转至硝化纤维素膜,6%脱脂奶粉进行封闭。随后分别以兔抗小鼠GAPDH (1:5000,SAB,#21612,美国),兔抗小鼠半胱氨酸天冬氨酸蛋白水解酶1 (cysteinyl aspartate specific proteinase-1,Caspase-1;1:2000,Origin,#TA323204S,美国),兔抗小鼠凋亡相关斑点样蛋白ASC型(apoptosis-associated speck-like protein containing a CARD,ASC;1:2000,Origin,#TA326289S,美国)的一抗4℃孵育过夜。次日复温后TBST洗膜5 min × 5次,在室温下孵育山羊抗兔辣根过氧化物酶结合的二级抗体(1:5000;ZSGB biotechnology,中国) 1 h。TBST洗膜5 min × 5次后,加入化学发光剂(碧云天,中国)后暗室曝光,使用成像系统(上海勤翔科技,中国)采集分析Caspase-1及ASC条带积分光密度值,其与相应GAPDH条带之比作为蛋白相对含量。
2.8. ELISA检测
术后24 h,小鼠在深麻醉下经心尖穿刺取血,同时快速断头取脑,液氮冻存。将血液于4℃下以3000 r/min的速度离心15 min吸取血清;取损伤半暗带皮质匀浆处理后于4℃下400 g离心5 min,吸取上清液。按说明书描述的方式,用ELISA试剂盒分别检测血清和损伤半暗带皮质中IL-1β和IL-18水平。
2.9. 改良神经功能评分(Modified Neurological Severity Score, mNSS)和体重变化率
在术后第1~7天,采用mNSS [10] 评分分别对每组小鼠进行神经功能评估,内容包括提尾试验、置地活动试验、平衡木试验、反射及反常运动试验等,并记录评分总和,用于反映小鼠神经功能损伤情况。同时记录小鼠术前体重为初始体重,术后1~7天每日同一时刻记录体重情况为当日体重。按照以下公式计算小鼠体重变化率:体重变化率 = 当日体重/初始体重 × 100%。
2.10. 统计分析
采用SPSS22.0与GraphPad Prism8.3软件进行统计学分析。正态分布计量资料采用均数 ± 标准差(
)表示,组间比较采用双尾t检验;非正态分布计量资料组间比较采用U检验;连续测量数据采用重复测量方差分析,单日组间比较采用单因素方差分析。P < 0.05表示差异有统计学意义。
3. 结果
3.1. 脑含水量
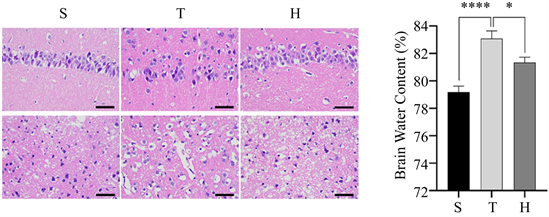
如图1(b)所示,与S组比较,T组脑组织含水量明显增加(****P < 0.0001),与T组比较,H组脑组织含水量明显降低(*P < 0.05)。
 (a) (b)
(a) (b)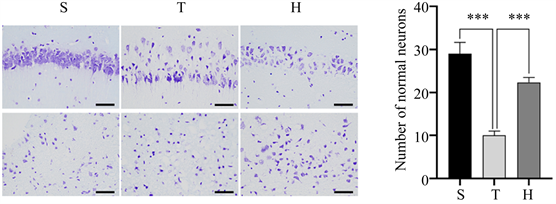 (c) (d)注:S组为假手术组,T组为创伤性脑损伤组,H组为分子氢治疗组。
(c) (d)注:S组为假手术组,T组为创伤性脑损伤组,H组为分子氢治疗组。
Figure 1. Brain water content and pathological changes of mice in three groups 48 h after surgery. (a) HE staining, ×400; (b) Brain water content of mice in three groups, compared with group S, ****P < 0.0001, compared with group H, *P < 0.05; (c) N Nissl staining, ×400; (d) The number of normal Nissl bodies per unit area of injured penumbra cortex in three groups of mice, compared with group S, ***P < 0.001, compared with group H, ***P < 0.001
图1. 3组小鼠术后48 h脑含水量及病理改变情况。(a) HE染色,×400;(b) 3组小鼠脑含水量情况,与S组比较,****P < 0.0001,与H组比较,*P < 0.05;(c) 尼氏染色,×400;(d) 3组小鼠损伤半暗带皮质单位面积下正常尼氏小体数量,与S组比较,***P < 0.001,与H组比较,***P < 0.001
3.2. 脑组织病理改变
脑组织HE染色示,S组细胞排列致密、整齐,细胞核完整,细胞质均匀丰富;与S组相比,T组细胞肿胀,间隙增宽,部分神经元可见核固缩与炎细胞浸润。相比于T组,H组的病理改变有所减轻(图1(a))。脑组织尼氏染色可见,S组包含大量形态正常的尼氏小体;T组尼氏小体数量减少,排列松散,可见大量异常尼氏小体。相比于T组,H组异常尼氏小体数量减少,神经元损伤减轻(图1(c),图1(d))。
3.3. Western Blot
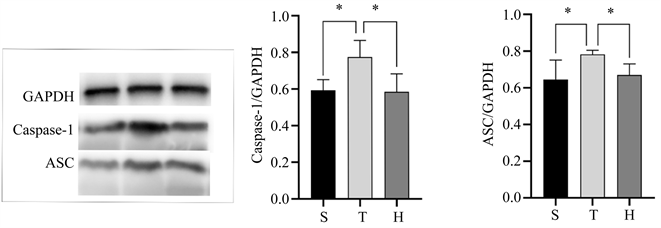
术后24 h脑组织细胞焦亡相关蛋白表达情况如(图2)所示,与S组相比,T组脑组织中Caspase-1、ASC表达水平均明显增加(均P < 0.05);与T组比较,H组脑组织中Caspase-1、ASC表达水平相对显降低(均P < 0.05)。
3.4. ELISA
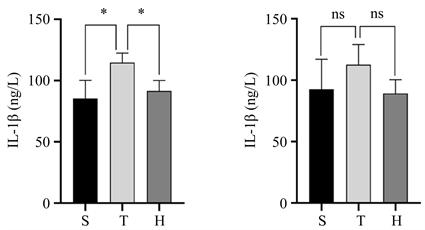
术后24 h血清和损伤半暗带皮质中IL-1β和IL-18表达水平如(图3)所示,与S组比较,T组血清中IL-1β和IL-18及损伤半暗带皮质中IL-18表达水平升高(均P < 0.05),与T组比较,H组血清中IL-1β和IL-18及损伤半暗带皮质中IL-18表达水平有所降低(均P < 0.05)。
3.5. mNss评分和体重变化率
术后7日mNSS评分结果(图4(a))显示,S组神经功能评分趋于一致,未见明显异常;T组术后即刻显示神经功能明显异常,同时具有缓慢自愈倾向;与T组比较,H组快速显示正向神经转归效应,并显著促进恢复速度,加快自愈趋势。
3组动物术后7日体重变化情况(图4(b))显示,S组小鼠体重增长趋势较为平稳,T组小鼠体重在术后出现大幅度降低,约96 h降低至最低水平,随后缓慢回弹;H组术后体重减轻幅度较小,且更早(约72 h)降低至最低点,并以较快的速度回升。
 (a) (b) (c)注:S组为假手术组,T组为创伤性脑损伤组,H组为分子氢治疗组。
(a) (b) (c)注:S组为假手术组,T组为创伤性脑损伤组,H组为分子氢治疗组。
Figure 2. Comparison of Caspase-1 and ASC protein contents in the injured penumbra cortex of mice in three groups 24 h after surgery. (a) Image of Caspase-1 and ASC protein contents in the injured penumbra cortex of mice in three groups; (b) Comparison of Caspase-1 expression levels in the injured penumbra cortex of the three groups of mice, compared with group S, *P < 0.05, compared with group H, *P < 0.05; (c) Comparison of ASC expression levels in the injured penumbra cortex of the three groups of mice, compared with group S, *P < 0.05, compared with group H, *P < 0.05
图2. 3组小鼠术后24 h损伤半暗带皮质Caspase-1、ASC蛋白含量的比较。(a) 3组小鼠损伤半暗带皮质两种蛋白显影情况;(b) 3组小鼠损伤半暗带皮质Caspase-1表达水平的比较情况,与S组比较,*P < 0.05,与H组比较,*P < 0.05;(c) 3组小鼠损伤半暗带皮质ASC表达水平的比较情况,与S组比较,*P < 0.05,与H组比较,*P < 0.05
 (a) (b)
(a) (b)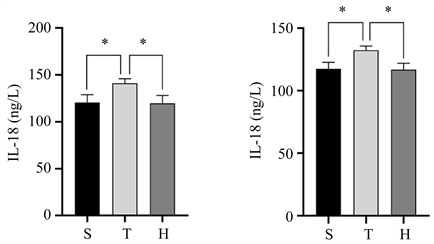 (c) (d)注:S组为假手术组,T组为创伤性脑损伤组,H组为分子氢治疗组。
(c) (d)注:S组为假手术组,T组为创伤性脑损伤组,H组为分子氢治疗组。
Figure 3. Expression levels of IL-1β and IL-18 in serum and injured penumbra cortex of the three groups of mice 24 h after surgery. (a) Comparison of serum IL-1β expression levels, compared with group S, *P < 0.05, compared with group H, *P < 0.05; (b) The expression level of IL-1β in the injured penumbra cortex showed no statistical significance compared with group S and group H; (c) Comparison of serum IL-18 expression levels, compared with group S, *P < 0.05, compared with group H, *P < 0.05; (d) IL-18 expression in the injured penumbra cortex compared with group S, *P < 0.05, compared with group H, *P < 0.05
图3. 三组小鼠术后24h血清和损伤半暗带皮质中IL-1β和IL-18表达水平。(a) 血清中IL-1β表达水平的比较情况,与S组比较,*P < 0.05,与H组比较,*P < 0.05;(b) 损伤半暗带皮质中IL-1β表达水平的比较情况,与S组比较,差异无统计学意义,与H组比较,差异无统计学意义;(c) 血清中IL-18表达水平的比较情况,与S组比较,*P < 0.05,与H组比较,*P < 0.05;(d) 损伤半暗带皮质中IL-18表达水平的比较情况,与S组比较,*P < 0.05,与H组比较,*P < 0.05
 (a) (b)注:S组为假手术组,T组为创伤性脑损伤组,H组为分子氢治疗组。
(a) (b)注:S组为假手术组,T组为创伤性脑损伤组,H组为分子氢治疗组。
Figure 4. Long-term survival of mice in three groups. (a) The mNSS score changing trend of miceinthree groups till the 7th day after surgery; (b) Body weight changes of mice in three groups till the 7th day after surgery
图4. 3组小鼠长期生存情况。(a) 3组小鼠术后1~7 d mNss评分变化趋势;(b) 3组小鼠术后1~7 d体重变化情况
4. 讨论
TBI是我国致死、致残率较高的疾病之一,患者常伴有神经转归差,预后不良等改变 [11]。在以往的研究中,该疾病经典的动物模型建立方法包括自由落体冲击(weight-drop, WD)、皮层冲击损伤(controlled cortical impact, CCI)和液压冲击损伤(fluid percussion injury, FPI)等 [12]。相较于前两种方法,FPI产生的冲击压力及时间具备一定的可控性,并且能够较好地模拟临床上TBI患者的神经功能障碍情况,独特的优势促使其成为TBI相关动物实验研究的一线建模方法。本次研究选取FPI法制备TBI模型,能够准确模拟TBI损伤特性,保证了研究动物神经转归稳定性。此外,区别于以往大量研究,本次实验选取了小鼠作为实验动物进行探索。虽然大鼠具备体积大、易于操作、存活率高等特性,是以往TBI相关研究的常用实验动物,但在基因及分子机制层面而言,小鼠与人类的同源性远高于大鼠,且小鼠的研究具备更好的转化特质,为保证本次研究结果能够具备临床转化优势,我们选取较为少见的TBI小鼠作为实验对象,同时应用改良的FPI法,进一步保证模型稳定。
近年来,分子氢已被证明对多种疾病具有改善作用 [13],其应用方式同样具备多样性。饮用富氢生理盐水的吸收途径并不能完全发挥分子氢的优势,肌肉注射效果又较为缓慢和局限,而静脉滴注创伤性较大,为此,我们选择吸入的方式探索分子氢的效用。与此同时,鉴于分子氢明显的剂量依赖性,以及越来越多的研究发现低浓度氢气并不理想的治疗效应,故而本次研究选择吸入较高浓度的氢气进行研究 [14]。本研究发现,分子氢对小鼠急性期脑水肿、远期生长情况和神经转归均具备明显改善作用,进一步证实了分子氢的神经保护效应。通过与课题组前期研究对比,我们还发现一个有趣的现象。在TBI大鼠层面,分子氢对体重变化的改善作用十分可观 [15],这可能与其促进胃肠道功能有关;但是在小鼠层面,体重变化相则对平稳,我们分析,这可能与小鼠体重基数较小,以及中枢神经系统差异有关。相应的,TBI小鼠的体重变化趋势似乎更加贴合临床TBI患者,这也进一步增加了本研究的转化优势 [16]。
此外,本次研究进一步探索了分子氢的神经保护作用机制。TBI过程伤常伴随着大量炎症因子释放 [17],创伤区的炎性浸润将进一步使炎性反应级联放大,导致神经功能异常。而细胞焦亡过程是TBI进展过程中的重要因素,也是导致该疾病预后不良的主要原因。在外界信号的刺激下,接头蛋白ASC与Caspase-1的前体结合并将其活化,一方面直接诱导细胞破裂,胞膜穿孔,内容物释放,导致炎症反应;同时,其对IL-1β和IL-18前体进行切割,将有活性的IL-1β和IL-18释放到胞外,导致炎症细胞募集,扩大炎症反应。在细胞焦亡过程中,神经细胞的结构破坏和炎症因子向胞外释放,对于脑组织原发病灶和全身性炎症反应均产生明显影响。而本实验对于损伤侧半暗带皮质和血清内同时进行的经典细胞焦亡相关因子检测进一步证实了分子氢的神经保护作用与抑制细胞焦亡途径有关。
与此同时,在常规检测血清中IL-1β和IL-18水平的同时 [18],本次研究也检测了脑组织中的炎症因子水平进行对比,结果显示,同一小鼠脑组织中炎症因子的下调水平高于血清。这也是对分子氢治疗作用的靶向性进行了一次探索。相比于全身性的抗炎效应,分子氢在原发病灶的治疗效果或许更佳,这可能与分子氢作为小分子,易于通过血脑屏障有关。对于术后24h损伤半暗带皮质中IL-1β水平差异无统计学意义的情况,可能与该取材时间点,炎症反应已由局部病灶级联放大至全身有关。与以往研究 [19] 不同的是,本次实验的治疗方式为长期、连续的吸入,并且将第一次吸入分子氢的时间设定为术后即刻开始,从结果上来看,神经功能和体重变化率曲线恢复的时间节点出现得更早,治疗效果更佳。这种设计既符合TBI临床患者多为重患的特点 [20],也反映了分子氢治疗效果的时效性,早期治疗能够更好地逆转急性期改变。而本研究选择了42%的吸入浓度,这即对分子氢动物实验的安全性及能否安全地应用于临床提出了挑战。
而同时,本研究应用了气体浓度监测装置,在安全距离为0.5 m的金属防爆箱内使用闭合气体回路的条件下进行实验,保证了研究的安全性。同时,2019年的COVID相关研究同样证实了高浓度(66.6% H2-33.3% O2)的分子氢对COVID及其肺损伤具有治疗作用,并已安全应用于临床 [21],这也进一步为本次研究结果向临床转化提供基础。然而,本实验也具有一定的局限性,即在分组中并未设置多个浓度梯度,没有对分子氢的最佳使用浓度进行标准性优化,同时缺少了描述细胞焦亡过程更为直观的扫描电镜检测,这也是本次研究有待改进之处。
5. 总结
综上所述,分子氢可以改善TBI小鼠脑水肿及神经转归,其机制可能与抑制细胞焦亡与全身炎症相关。
NOTES
*通讯作者。