摘要: 目的:总结分析结直肠息肉相关的常见血清学指标,研究癌变高危因素。方法:回顾性分析青岛大学附属医院2171例结直肠息肉患者,分为息肉组、癌变组,分别1820例、351例。收集各组病例的血清学指标和BMI进行统计学分析。结果:癌变组和息肉组在LDL-C、TC、TG、ALP、CEA、BMI、NLR方面差异有统计学意义(P < 0.05);其中LDL-C、TC、TG、BMI方面,癌变组中位数明显低于息肉组,ALP、CEA、NLR方面,癌变组中位数明显高于息肉组。Logistic回归分析发现,LDL-C、CEA、NLR为结直肠息肉癌变的独立危险因素;Spearman相关性分析显示LDL-C与结直肠息肉癌变呈负相关,CEA、NLR与结直肠息肉癌变呈正相关;绘制ROC曲线,LDL-C的AUC为0.5778,取<2.435为最佳截断值,敏感度为39.32%,特异度为74.62%;CEA的AUC为0.7306,取>2.685为最佳截断值,敏感度为64.10%,特异度为72.42%;NLR的AUC为0.6947,取>2.215为最佳截断值,敏感度为51.57%,特异度为79.51%。联合诊断时虽然AUC为0.6734,无明显的改善,但敏感度明显升高。结论:较低的低密度脂蛋白胆固醇、较高的粒淋比和癌胚抗原是结直肠息肉癌变的高危因素,可作为结直肠息肉癌变的预测指标,三者联合诊断可明显提高其敏感性。
Abstract:
Objective: To summarize and analyze the common serological indexes related to colorectal polyps and study the high-risk factors of canceration. Methods: 2171 patients with colorectal polyps in the Affiliated Hospital of Qingdao University were analyzed retrospectively. They were divided into polyp group and canceration group, 1820 cases and 351 cases respectively. The serological indexes and BMI of each group were collected for statistical analysis. Results: There were significant differences in LDL-C, TC, TG, ALP, CEA, BMI and NLR between canceration group and polyp group (P < 0.05); in terms of LDL-C, TC, TG and BMI, the median of canceration group was significantly lower than that of polyp group. In terms of ALP, CEA and NLR, the median of canceration group was significantly higher than that of polyp group. Logistic regression analysis showed that LDL-C, CEA and NLR were independent risk factors for colorectal polyp canceration; Spearman correlation analysis showed that LDL-C was negatively correlated with colorectal polyp canceration, and CEA and NLR were positively correlated with colorectal polyp canceration; plot the ROC curve: The AUC of LDL-C was 0.5778, and <2.435 was the best cut-off value. The sensitivity was 39.32% and the specificity was 74.62%; the AUC of CEA was 0.7306, and >2.685 was the best cut-off value. The sensitivity was 64.10% and the specificity was 72.42%; the AUC of NLR is 0.6947, and >2.215 is the best cutoff value. The sensitivity is 51.57% and the specificity is 79.51%. Although the AUC of the combined diagnosis was 0.6734, no significant improvement, but a marked increase in sensitivity. Conclusion: Low LDL cholesterol, high granulocyte to lymph ratio and carcinoembryonic antigen are high risk factors for colorectal polyp canceration, which can be used as predictors of colorectal polyp canceration. The combined diagnosis of the three can significantly improve its sensitivity.
1. 引言
结直肠癌是最常见的恶性肿瘤之一,2020年其发病率在全球居于恶性肿瘤第3位(10.0%),死亡率高居第2位(9.4%),是占全球发病和死亡首位的消化系统恶性肿瘤 [1]。对于结直肠癌的预后,I期结直肠癌患者预后较好,5年生存率超过91% [2],IV期结直肠癌患者即使手术切除,5年生存率仅12% [2],因此对于做好结直肠癌的早期筛查尤为重要,虽然结肠镜对结直肠癌筛查的发挥了重要的作用 [3],但毕竟是侵入性操作,应当尽量较少不必要的操作。本研究选取了青岛大学附属医院的部分病例资料,评估常见血清学指标对结直肠息肉癌变的预测价值,为结直肠息肉患者诊疗计划的制定提供理论支持。
2. 资料与方法
2.1. 一般资料
选取青岛大学附属医院2013-05-01至2020-08-31期间收治的资料齐全结直肠息肉患者,共2171例,其中男1396例(64.3%),女775例(35.7%),年龄(62.86 ± 10.75)岁;根据息肉组织病理结果中有无癌变,分为息肉组、癌变组,息肉组1820例(男1163例,女657例),癌变组351例(男233例,女118例)。
2.2. 研究方法
2.2.1. 纳入标准
1) 所有患者均行完整的电子结肠镜检查;
2) 临床特征、镜下病理特点以及病理资料完整;
3) 肠道准备充分,不影响结直肠镜对息肉根部的观察;
4) 无明显影响炎性指标的病变:如急性和化脓性感染、各种中毒、组织损伤、恶性肿瘤、急性大出血、急性溶血、某些传染病、血液病、自身免疫性疾病、缺铁性贫血、脾切除术等。
2.2.2. 排除标准
1) 电子结肠镜未检查至回盲部;
2) 孕妇、哺乳期患者、家族性腺瘤性息肉病、侧向发育型息肉患者;
3) 病例资料不全的患者;
4) 肠道准备不充分,影响观察的患者;
5) 存在明显炎性病变:如急性和化脓性感染、各种中毒、组织损伤、恶性肿瘤、急性大出血、急性溶血、某些传染病、血液病、自身免疫性疾病、缺铁性贫血、脾切除术等。
2.2.3. 数据收集
经电子肠镜检查及活检病理确诊的2171例结直肠息肉患者,记录其中性粒细胞/淋巴细胞比值(Neutrophil to lymphocyte ratio, NLR) (以下简称为粒淋比)、乳酸脱氢酶(Lactate dehydrogenase, LDH)、碱性磷酸酶(Alkaline phosphatase, ALP)、总胆固醇(Total cholesterol, TC)、甘油三脂(Triglyceride, TG)、低密度脂蛋白胆固醇(Low density lipoprotein cholesterol, LDL-C)、癌胚抗原(Carcinoembryonic antigen, CEA)、体质指数(Body mass index, BMI)。
2.3. 统计方法
运用SPSS25.0对各病例组进行统计学分析,非正态分布的计量资料以M (P25, P75)表示。癌变组和息肉组间比较采用Mann-Whitney U检验(P < 0.05),相关性分析采用Spearman秩相关分析。采用Logistic回归分析息肉癌变的独立危险因素(息肉组赋值为0,癌变组赋值为1),预测价值评估采用受试者工作特征(ROC)曲线分析。以P < 0.05差异有统计学意义。
3. 结果
3.1. 癌变组和息肉组的基本资料
经K-S检验,病例组和对照组各样本指标中除了癌变组BMI外均为非正态分布,经过Mann-Whitney U分析,两组间在LDL-C、TC、TG、ALP、CEA、BMI、NLR方面差异有统计学意义(P < 0.05),而在LDH方面两组差异无统计学意义(Z = −0.677, P = 0.501);其中在LDL-C、TC、TG、BMI方面,癌变组中位数(2.70 vs 2.96、4.57 vs 4.98、1.04 vs 1.29、24.51 vs 24.90)均明显低于息肉组,并且两组间差异有统计学意义(P < 0.05);在ALP、CEA、NLR方面,癌变组中位数(69.00 vs 65.00、3.45 vs 1.83、2.27 vs 1.60)明显高于息肉组,差异有统计学意义(P < 0.05);详见表1和表2。

Table 1. Comparison of canceration group and polyp group
表1. 癌变组和息肉组的比较

Table 2. Comparison of polyp group and canceration group
表2. 息肉组和癌变组的比较
3.2. Logistic回归分析结直肠息肉癌变的相关因素
对结直肠息肉癌变的相关因素进行Logistic回归分析发现,LDL-C、TC、TG、CEA、NLR是结直肠息肉癌变的独立影响因素,其中TC、TG是结直肠息肉癌变的保护性因素(OR < 1),LDL-C、CEA、NLR为结直肠息肉癌变的独立危险因素(OR > 1)。详见表3。

Table 3. Logistic regression analysis of the impact of related indicators on colorectal polyp canceration
表3. Logistic回归分析相关指标对结直肠息肉癌变的影响
3.3. 癌变组和息肉组间相关性分析
对结直肠息肉癌变的高危因素进行相关性分析显示,LDL-C与结直肠息肉癌变呈负相关(r = −0.009, P < 0.05),CEA (r = 0.294, P < 0.05)、NLR (r = 0.248, P < 0.05)与结直肠息肉癌变呈正相关。详见表4。

Table 4. Correlation analysis of LDL-C, CEA, NLR between canceration group and polyp group
表4. 癌变组和息肉组间LDL-C、CEA、NLR的相关性分析
3.4. 息肉组和癌变组的受试者工作曲线(ROC曲线)
以息肉组作为对照,将351例癌性息肉患者的LDL-C绘制ROC曲线,结果显示:LDL-C的ROC曲线下面积(AUC)为0.5778 (95% CI 0.5445~0.6111, P < 0.0001),取<2.435为最佳截断值,敏感度为39.32%,特异度为74.62%。详见图1。
以息肉组作为对照,将351例癌性息肉患者的CEA绘制ROC曲线,结果显示:CEA的ROC曲线下面积(AUC)为0.7306 (95% CI 0.6992~0.7619, P < 0.0001),取>2.685为最佳截断值,敏感度为64.10%,特异度为72.42%。详见图2。
以息肉组作为对照,将351例癌性息肉患者的NLR绘制ROC曲线,结果显示:NLR的ROC曲线下面积(AUC)为0.6947 (95% CI 0.6631~0.7263, P < 0.0001),取>2.215为最佳截断值,敏感度为51.57%,特异度为79.51%。详见图3。

Figure 1. ROC curve of LDL-C in polyp group and canceration group
图1. 息肉组和癌变组关于LDL-C的ROC曲线
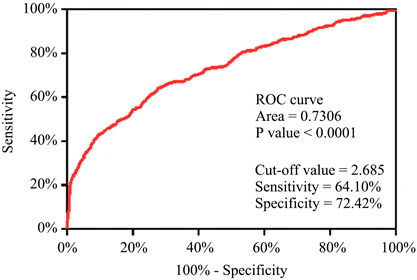
Figure 2. ROC curve of CEA in polyp group and canceration group
图2. 息肉组和癌变组关于CEA的ROC曲线

Figure 3. ROC curve of NLR in polyp group and canceration group
图3. 息肉组和癌变组关于NLR的ROC曲线
3.5. 各指标联合诊断的效能
以LDL-C、CEA、NLR各自的截断值界定为“阳性”,其中有一项阳性即定义联合诊断结果为阳性,以此分析各指标联合诊断的临床价值。其中单项指标的AUC分别为0.5697, 0.6826, 0.6554;联合诊断时敏感性明显升高(88.03% vs 39.32%、64.10%、51.57%),但AUC为0.6734,并无明显的改善。详见表5和图4。

Table 5. Diagnostic value of combined diagnosis of various indicators for colorectal polyp canceration
表5. 各指标联合诊断对结直肠息肉癌变的诊断价值

Figure 4. ROC curve of combined diagnosis of various indicators
图4. 各指标联合诊断的ROC曲线
4. 讨论
中国目前结直肠癌负担重 [4],呈现发病率、死亡率双高的特征,亟待提高结直肠癌早期筛查能力 [4]。而结直肠息肉癌变主要通过三个主要途径产生,包括腺瘤–癌序列、锯齿状途径和炎症途径 [5],其中结直肠腺瘤约占结直肠腺癌来源的85%~95% [6],本次研究的主要对象是符合腺瘤–癌序列的结直肠息肉,并根据其病理类型分为息肉组、癌变组。
在血脂分析常见指标的比较中,癌变组LDL-C明显低于息肉组,较低的LDL-C被认为是结直肠息肉癌变的独立危险因素,虽然Fang等 [7] 未发现血清高密度胆固醇、总胆固醇、甘油三酯和载脂蛋白A和B等血清脂质谱与结直肠癌风险的相关性,这与本研究基本相符;但是LDL-C与结直肠息肉癌变的相关性也未得到证实 [7] [8],且Liu等 [9] 发现TG水平升高是晚期腺瘤患者息肉复发的独立风险预测因子,这与本研究不甚相符,可能与不同地区生活习惯的差异、检验方法的差异有关,有待进一步研究。目前也有研究发现了新型脂质生物标志物,如聚酮脂质(810.1) [10]、血浆外泌体脂质 [11] 可作为结直肠息肉癌变的生物标志物,有待进一步探索研究。
在粒淋比方面,癌变组NLR中位数明显高于息肉组,并且和息肉癌变与否成正相关,NLR > 2.215可能是结直肠息肉癌变的独立危险因素,结合相关研究,中性粒细胞能够释放氧自由基、趋化因子、细胞生长因子蛋白酶等成分,并激活核因子-κB,从而促进肿瘤细胞生长 [12],并且肿瘤中淋巴细胞浸润的增加与癌症患者对细胞毒性治疗更好的预后相关 [13],而NLR能够反映全身炎性反应情况 [14],NLR已被证实与多种恶性肿瘤相关,NLR升高被认为是各种癌症的不良预后指标 [15]。一项Mete分析显示在许多实体瘤中,高NLR与不利的总生存率相关 [16]。而对于与结直肠病变的相关性,吕智豪等 [17] 发现结直肠癌患者的NLR明显高于息肉患者或健康人群,这与本研究一致,另有研究认为NLR对结直肠癌预后评估 [14] [18]、化疗后细菌感染的评估 [15] 有一定的意义,也有研究认为血小板–中性粒细胞与淋巴细胞比率可作为转移性结直肠腺癌预后工具 [19],但是关于NLR对结直肠息肉癌变的预测性研究仍较少,有待进一步研究。
在CEA的比较中,CEA与结直肠息肉癌变呈正相关性,癌变组中位数也明显高于息肉组,被认为是结直肠息肉癌变的高危因素,对于CEA的应用在临床上已经得到了证实 [20],其对结肠癌评估的敏感性明显高于CA24-2、CA19-9,是一种非常有前景的靶向生物标志物 [21],CEA还通过多种方式促进了结直肠癌的进展和转移。CEA保护转移细胞免于死亡,改变血窦微环境,促进粘附分子的表达和恶性细胞存活,并且是促血管生成分子 [21],还被认为是评估化疗效果的有效指标 [22]。
在与结直肠息肉癌变相关的LDL-C、CEA、NLR指标的临床诊断价值分析中发现,三者联合诊断可以明显提高结直肠息肉癌变诊断的敏感性,但曲线下面积(AUC)未见明显改善,关于此方面的研究较少,有待进一步研究。
5. 结论
较低的低密度脂蛋白胆固醇、较高的粒淋比和癌胚抗原是结直肠息肉癌变的高危因素,可以作为结直肠息肉癌变的预测指标,三者联合诊断可明显提高其敏感性。
声明
该研究参与患者知情同意,并经青岛大学附属医院伦理委员会批准通过。
NOTES
*第一作者。
#通讯作者Email: liuxishuang1@sina.com