1. 引言
pH和细胞、组织的多种功能相关,包括离子通道 [1] [2] 、内吞作用 [3] [4] [5] 、细胞增殖和凋亡 [6] 、肌肉收缩等 [7] [8] [9] 。pH稳态是蛋白保持正常结构和功能,实现生命体正常运转的关键 [10] 。肿瘤、阿尔兹海默症等多种疾病与pH异常密切相关。据报道,不同的肿瘤组织,其手术切除不完全的发生率在25%~40%之间 [11] 。近年来,临床上尝试用荧光探针特异性识别肿瘤细胞为手术导航,提高手术治愈率。受肿瘤异质性等因素限制,靶向特异性受体或酶的探针不具有普适性。酸化是所有实体肿瘤微环境的特征性标志之一 [12] [13] ,因此pH荧光探针具备识别各种肿瘤组织的可能。
上转换发光材料有反斯托克斯位移的特点,近红外光激发,可见光发射,在生命体成像中具有独特的优势,包括成像深度大、不受背景荧光干扰、半峰宽窄、发光稳定性好等 [14] [15] [16] 。同时,肿瘤组织的高通透性和滞留效应有助于上转换纳米颗粒(UCNPs)在肿瘤中的聚集和成像 [17] [18] 。上转换材料本身不具备识别能力,作为荧光供体与带有识别基团的荧光受体组装形成荧光探针,发光共振能量转移(LRET)效应是构筑这类探针的常用策略。目前基于上转换发光材料开发的pH探针通常在一个波长下产生LRET效应 [19] [20] ,多个波长下的LRET过程有助于提高探针灵敏度,这类探针鲜有报道。
前期开发的有机小分子荧光探针CouDa在酸性条件下580 nm处有强吸收,碱性环境下探针与OH−发生加成反应,最大吸收峰蓝移到430 nm,能检测细胞及小鼠肿瘤中pH变化,但响应时间较长,灵敏度不高 [21] 。UCNPs (NaYF4: 20% Yb, 2% Er)的发射峰与CouDa吸收峰位置吻合,在409 nm、542 nm和656 nm三个波长下都可能产生LRET效应,且随pH变化的趋势相反,有助于提高检测灵敏度(图1)。通过合理修饰,UCNPs表面可以形成疏水层,减弱水分子对OH−的作用,可能加速CouDa与OH−的反应。据此,我们在包裹了两亲聚合物C18PMH-mPEG的UCNPs (NaYF4: 20% Yb, 2% Er)表面负载CouDa,得到UCNPs@CouDa探针。环境pH由酸性变成碱性时,410 nm处上转换发光(UCL410 nm)减弱,同时UCL542 nm和UCL656 nm增强。在pH 6.0~8.5之间,上转换发光比值UCL542 nm/UCL409 nm与pH的变化呈线性关系,pKa值约7.3,对pH的响应选择性高,适用于生命体相关的pH成像。

Figure 1. (a) Imaging illustration of UCNP@CouDa toward pH fluctuations; (b) UCL spectra of UCNPs (NaYF4: 20% Yb, 2% Er), absorption spectra of CouDa in acidic and basic environment, and the reaction mechanism of CouDa with OH−
图1. (a) UCNPs@CouDa对pH变化的响应示意图;(b) UCNPs (NaYF4: 20% Yb, 2% Er)发射光谱图,CouDa在酸/碱性环境中吸收光谱图,及CouDa对pH响应机理示意图
2. 实验部分
2.1. 仪器与试剂
Y2O3 (99.99%), Yb2O3 (99.99%), Er2O3 (99.99%), CF3COOH (99.5%),油胺(>40%, GC)购自梯希爱(上海)化成工业发展有限公司,mPEG-NH2 (MW = 5000 Da)购自上海阿拉丁生化科技有限公司,C18PMH-COOH (MW = 30,000~500,00 Da)购自Aldrich公司。合成所用试剂和溶剂都是国内供货商提供的分析纯等级,使用时没有进一步提纯。实验用水为Milli-Q超纯水。上转换荧光光谱在北京卓立汉光ZolixScan ZLX-UPL光谱仪上测定(980 nm激光光源),其余荧光光谱在Horiba FluoroMax-4荧光光谱仪上测定。吸收光谱用Perkin Elmer Lambda 35紫外可见光谱仪测定。溶液pH用PHS-3精密pH计测定。X射线衍射数据在Simadzu XRD-6000 diffractometer上采集,透射电镜数据由JEOL JEM-2100收集。
2.2. 合成及表征
2.2.1. C18PMH-mPEG合成及表征
参照文献合成 [22] 。在含有C18PMH (40 mg, 1 eq.), mPEG-NH2 (572 mg, 1 eq.), EDC·HCl (4 mg)的烧瓶中加入CH2Cl2 (20 mL)和Et3N (24 µL),N2保护下室温避光搅拌24 h。停止反应,N2吹干,加入二次水搅拌溶解,转移到半透膜中(M = 14 k),透析48小时(期间多次换水),冷冻干燥。经核磁表征,mPEG对C18PMH的修饰率为100%。
1H NMR (400 MHz, CDCl3) δ: 3.53-3.80 (m, br, CH2 of mPEG), 1.28 (br, CH2 of C18PMH), 0.91 (br, CH3 of C18PMH).
2.2.2. UCNPs合成
UCNPs (NaYF4: 20% Yb, 2% Er)参照文献合成 [23] 。(CF3COO)3M (M = Y, Yb, Er)由相应氧化物溶于CF3COOH和水的混合溶液(1/1,v/v)中制得。在20 mL油胺中分别加入CF3COONa (2 mmol, 285.60 mg), (CF3COO)3Y (0.78 mmol, 375.96 mg), (CF3COO)3Yb (0.20 mmol, 113.23 mg), (CF3COO)3Er (0.02 mmol, 11.21 mg),搅拌溶解,缓慢升温至120℃,抽真空2小时除去体系中的水和氧气后,向体系内通入高纯氩气,缓慢升温至340℃并反应2小时。停止反应,降至60℃以下并加入20 mL乙醇,10,000 r/min离心,粒子用1:1的正己烷/乙醇混合溶液和水分别洗涤三次,分散于正己烷中待用。透射电镜和X射线衍射表征。
2.2.3. UCNPs@C18PMH-mPEG合成
取上述UCNPs,超声分散于氯仿中,加入C18PMH-mPEG (200 mg),超声,搅拌过夜,室温下用N2吹干溶剂,加入纯水搅拌至分散,10,000 r/min离心,强烈超声水洗至无泡沫,过膜除掉大颗粒(200 µm),分散在水中4℃保存。透射电镜表征。
2.2.4. UCNP@CouDa合成
把UCNPs@C18PMH-mPEG (30 mg)分散在pH约5.0的Na2HPO4-柠檬酸缓冲溶液中(5 mL),滴加含CouDa (15 mg)的DMSO溶液(0.5 mL),避光剧烈搅拌过夜,离心,用含10%乙醇的上述酸性缓冲溶液超声洗涤,紫外–可见吸收光谱检测洗出液中没有CouDa为止。过膜滤掉大颗粒(200 µm),分散在酸性缓冲液中4℃保存。
2.2.5. CouDa合成
按照文献合成 [21] 。
2.2.6. CouDa负载量测定
按照文献方法 [24] ,称取一定量干燥的UCNPs@C18PMH-mPEG颗粒,依次用一定量的CH3Cl、CH3OH多次分散超声洗涤,所得溶液定容,用紫外–可见光谱仪检测吸光度,并与标准工作曲线对照,计算负载量。
2.3. 光谱测试方法
实验中所用缓冲溶液均为Na2HPO4-柠檬酸体系(0.2 M Na2HPO4,0.1 M柠檬酸),用NaOH和HCl调节pH值。保存在酸性溶液中的UCNPs@CouDa粒子离心后用二次水洗涤,分散到缓冲溶液中,pH计验证酸度。UCNPs@CouDa溶液调整到目标pH值后,放置30分钟左右待光谱稳定后收集数据。上转换发光光谱采集完成后,立即进行紫外–可见吸收光谱测试。
择性测试中,UCNPs@CouDa探针分散到pH 7.4的Na2HPO4-柠檬酸缓冲溶液中,加入各竞争性物种,放置30分钟左右待光谱稳定后采集数据。对照组不加其他物种。
3. 结果与讨论
3.1. UCNPs@CouDa的设计与表征
许多pH探针pKa值过于偏酸性,不适合用于生命体检测。CouDa探针具有适合于生命体检测的pKa值(7.4)和线性响应范围(6.5~8.5),但响应速率较慢 [21] 。基于OH−与CouDa亲核加成反应的机理,我们推测在水溶液中OH−与水分子相互作用阻碍了与CouDa的反应。当探针处于疏水环境中时,OH−受水分子影响减小,响应速率可能会加快。根据紫外–可见吸收光谱可知,碱性和酸性环境下,CouDa的最大吸收峰分别位于430 nm和580 nm附近,UCNPs (NaYF4: 20% Yb, 2% Er)的三个发射峰位于409 nm、542 nm和660 nm,三个波长下的发射光都可能被CouDa吸收发生LRET过程,且环境pH变化时三个波长下的LRET效应变化趋势相反,有利于提高探针响应灵敏度。因此用含有疏水和亲水长链的两亲聚合物C18PMH-mPEG包裹表面带有油胺的UCNPs (UCNP@OM),水溶液中粒子表面形成一个疏水层,带有C12烷基长链的CouDa通过疏水–疏水相互作用可自组装到这一疏水层中 [24] [25] ,加快与OH−的响应。
由热分解法制得UCNPs@OM颗粒,透射电镜及高分辨电镜显示粒径在20~30 nm左右,具有清晰的晶格条纹,晶格间距约0.51 nm,与β态UCNPs(100)晶面的晶格间距相吻合(图2(a),图2(b))。图2(c)是制得的UCNPs@OM粒子X射线衍射图(XRD)及报导的β态粒子标准卡(JCPDS File No. PDF 28-1192),对比发现晶体的衍射峰位置及强度与β态相吻合,同时也存在少量α态粒子(虚线标注)。C18PMH-mPEG包裹后,纳米颗粒形貌没有发生明显变化(图2(d))。
CouDa探针在UCNPs上的负载量通过紫外−可见吸收光谱测定。CouDa及C18PMH-mPEG通过疏水–疏水相互作用自组装到UCNPs表面,在可溶性有机溶剂中强烈超声时,它们会从颗粒表面脱落 [24] 。通过测量有机溶剂的吸光度并与标准工作曲线对照,测得每克UCNPs表面负载的CouDa为119毫克,即负载率为11.9%。
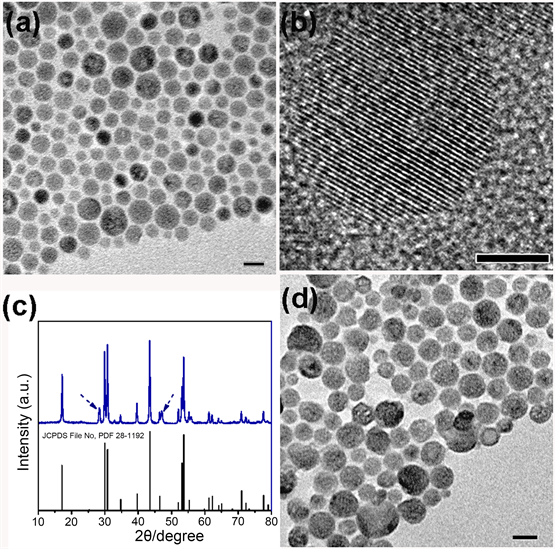
Figure 2. (a) TEM, (b) HR-TEM and (c) XRD spectra of UCNPs@OM; (d) TEM of UCNPs@C18PMH-mPEG. Scale bar, 20 nm ((a), (b)), 5 nm (b)
图2. UCNPs@OM粒子(a)透射电镜图,(b)高分辨透射电镜图,(c) XRD图,下图为β态粒子对照图,JCPDS File No. PDF 28-1192;(d) UCNPs@C18PMH-mPEG粒子透射电镜图。标尺:20 nm ((a), (b)), 5 nm (b)
3.2. UCNPs@CouDa光谱测试
首先测试了UCNPs@CouDa对pH的响应速率。在pH 7.4的缓冲溶液中,20分钟左右探针的上转换发射及紫外–可见吸收光谱都达到了稳定状态,说明探针与OH−的反应达到平衡,在pH 8.4的溶液中响应时间相近,这一速率远大于小分子探针CouDa的响应速率(pH 8.4时40 min左右) [21] ,表明疏水环境有利于CouDa对OH−的响应(图3)。
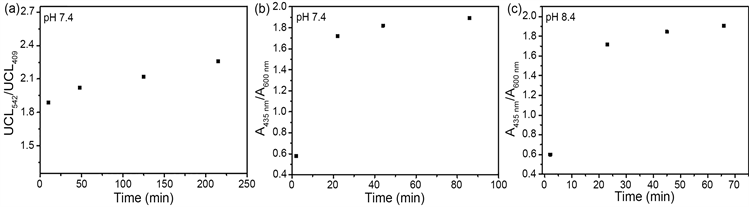
Figure 3. Time dependent upconversion luminescence spectra and absorption spectra of UCNPs@CouDa in Na2HPO4-citric buffer solutions with pH 7.4 ((a), (b)) and pH 8.4 (c)
图3. 在pH 7.4和8.4的Na2HPO4-柠檬酸缓冲溶液中UCNPs@CouDa的上转换发射光谱(a)及紫外–可见吸收光谱((b), (c))随时间变化图
生理pH条件下,UCNPs@CouDa探针的上转换发射光谱如图4(a)所示。pH 5.0左右探针在409 nm,542 nm及656 nm出现三组UCNPs的特征发射峰。当pH从5.0逐渐增大到8.2时,409 nm处上转换发射有所减弱,而542 nm和656 nm处上转换发射都显著增强,表明UCNPs三个波长下的发射光与CouDa之间都存在LRET过程。这与CouDa分子的吸收峰位置、强度随pH的变化相关。pH 5.0附近,CouDa在500~700 nm范围有一个强的宽吸收峰,当pH增大到8.0以上时,这一吸收峰减弱到几乎消失,同时在430 nm附近出现一个新的较弱的吸收峰(图4(c))。因此,酸性环境中UCNPs在542 nm和656 nm的上转换发射光被CouDa吸收,LRET效应较强,UCL542 nm和UCL656 nm较弱。随pH增大CouDa对这两处光的吸收减弱,LRET效应减弱,UCL542 nm和UCL656 nm逐渐增强。而409 nm处的变化趋势恰好相反,随pH增大,CouDa对此处光的吸收逐渐增强,LRET效应随之增大,导致UCL409 nm减弱。542 nm和409 nm处发射峰比值(UCL542 nm/UCL409 nm)对pH作图可以发现,酸度小于6.0时该比值几乎保持不变,pH 6.0 到8.5范围内两者间存在显著的线性关系,UCL542 nm/UCL409 nm比值随pH增大而快速增大,到pH 8.5时该比值增大约2.8倍(图4(b))。小分子探针CouDa对pH的线性响应范围在6.5~8.5 [21] ,负载到UCNPs的疏水层后,其线性响应范围拓宽,表明疏水环境有利于CouDa对pH的响应。与小分子探针CouDa相比,UCNPs@CouDa对pH的相应速率更快,线性响应范围更宽,对pH检测更加灵敏。通过Henderson-Hasselbalch方程 [26] 计算可得,UCNPs@CouDa探针的pKa值约7.3,与小分子探针CouDa的pKa值一致(图4(d))。

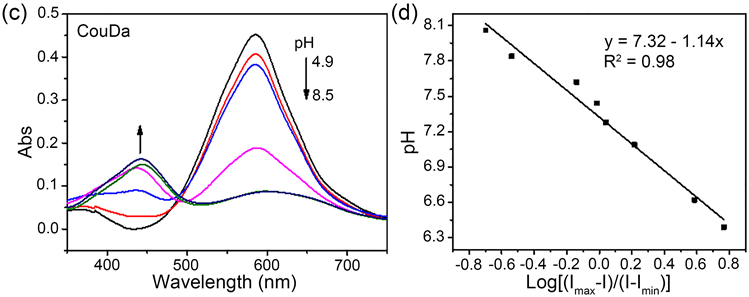
Figure 4. (a) Upconversion luminescence spectra of UCNPs@CouDa in Na2HPO4-citric buffer solutions with pH 5.1 to 8.5; (b) Luminescence ratio (UCL542 nm/UCL409 nm) versus pH, and (b inset) the linear fitting between pH 6.0 to 8.5; (c) Absorption spectra of CouDa with pH 4.9 to 8.5; (d) Linear fitting of Log[(Imax−I)/(I−Imin)] versus pH, where I is the ratio of UCL542 nm/UCL409 nm; λex = 980 nm
图4. (a) UCNPs@CouDa在pH 5.1到8.5的Na2HPO4-柠檬酸缓冲溶液中的上转换发射光谱图;UCL542 nm/UCL409 nm比值随pH的变化图(b),以及pH 6.0~8.5两者之间的线性关系拟合图(b内插入图);(c) pH 4.9~8.5小分子CouDa紫外–可见吸收光谱随pH变化图;(d) Log[(Imax−I)/(I−Imin)]和pH之间的线性关系拟合图,I为上转换发光比值UCL542 nm/UCL409 nm;λex = 980 nm
上转换发光检测的同时收集了UCNPs@CouDa探针的紫外–可见吸收光谱随pH的变化(图5)。pH 5.0左右的酸性环境中,上转换纳米探针在450~700 nm范围出现了宽而强的吸收峰,半峰宽明显大于小分子探针CouDa,表明负载在UCNPs表面疏水层中的CouDa可能部分聚集。pH逐渐增大时该吸收峰逐渐减弱,同时在440 nm附近出现新的吸收峰。pH小于6.0时440 nm和543 nm处的吸收强度比值变化很小(A440 nm/A543 nm),当pH大于6.0时,该比值随pH增大而增大,pH大于7.8时该比值不再变化,pH 6.0~7.8范围内线性关系不明显。因此与小分子探针CouDa相比,生理pH条件下UCNPs@CouDa的紫外–可见吸收光谱的灵敏度下降。

Figure 5. (a) Absorption spectra of UCNPs@CouDa in Na2HPO4-citric buffer solutions with pH 5.0 to 8.2, (b) The absorption ratio (A440 nm/A543 nm) versus pH
图5. UCNPs@CouDa在pH 5.0~8.2的Na2HPO4-柠檬酸缓冲溶液中的紫外–可见吸收图(a),409 nm 和542 nm处吸收比值(A440 nm/A543 nm)随pH的变化图(b)
接着对UCNPs@CouDa的选择性进行了测试。把上转换探针分散在pH 7.4的Na2HPO4-柠檬酸缓冲溶液中,加入各生命体常见物种。如图6所示,这些物种对探针的上转换发光影响很小,对上转换发光强度比值UCL542 nm/UCL409 nm的影响在0.1个单位以内。在pH线性响应范围6.0到8.5之间,该比值变化约2.8倍。可见UCNPs@CouDa对生理pH变化的响应具有高选择性。
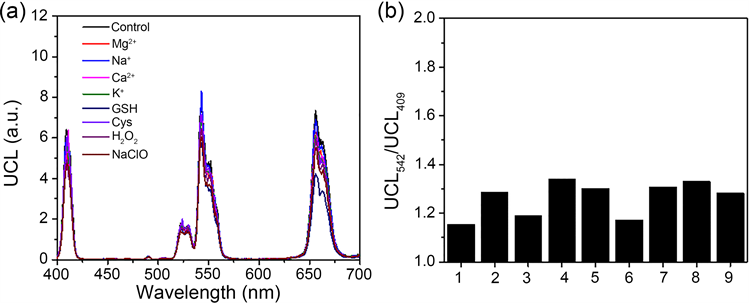
Figure 6. (a) Upconversion luminescence spectra of Na2HPO4-citric buffer solution in pH 7.4 containing UCNPs@CouDa and other biorelevant species. (b) Histograms of upconversion luminescence intensity ratio UCL542 nm/UCL409 nm in diagram a. 1. Control, without competitive species; 2. Mg2+; 3. Na+; 4. Ca2+; 5. K+; 6. GSH; 7. Cys; 8. H2O2; 9. ClO− (2~6, 10 mM; 6~7, 1 mM; 8-9, 200 µM). λex = 980 nm
图6. 含UCNPs@CouDa的pH 7.4缓冲溶液中(Na2HPO4-柠檬酸)加入各竞争性物种后的上转换发射图(a),及各物种存在时溶液的上转换发射光强度比值(UCL542 nm/UCL409 nm)。1、参照,不含竞争性物种,2、Mg2+,3、Na+,4、Ca2+,5、K+,6、GSH,7、Cys,8、H2O2,9、ClO− (2~6, 10 Mm; 6~7, 1 mM; 8~9, 200 µM)。λex = 980 nm
4. 结论
有机小分子CouDa负载到UCNPs@C18PMH-mPEG疏水层,组合成上转换探针UCNPs@CouDa。pH变化时,上转换颗粒的三组特征发射峰都可以被CouDa吸收,产生三重LRET效应,且409 nm处LRET变化趋势与542 nm及656 nm处相反,使得UCL409 nm和UCL542 nm、UCL656 nm变化趋势相反,单位pH范围内UCL542 nm/UCL409 nm比值增大,提高了检测灵敏度。UCNPs@CouDa探针的pKa值约7.3,对pH变化的线性响应范围在6.0~8.5,对pH检测具有高选择性。与小分子探针CouDa相比,负载到疏水层的CouDa对pH响应更迅速,线性响应范围更宽,表明疏水环境有利于这一亲核加成机理的pH检测。
基金项目
感谢国家自然科学基金(22107054)、南通市基础科学基金(JC2021114)和江苏省大学生创新训练计划项目(202110304132H)的资助。
参考文献
NOTES
*通讯作者。