摘要: 目的:分析胃癌淋巴结转移对不同T分期特异性生存率的影响。方法:以美国SEER数据库为基础,筛选出2010年至2015年信息完整的1918例胃癌手术患者,分析胃癌的特异性生存率(CSS)。生存分析采用K-M法,利用单变量和多变量Cox比例风险回归评估独立预后的生存风险因素,计算并校正特异性死亡的风险比(HR)结果,95%置信区间(CI)。组间逐对比较采用Bonferroni法。结果:胃癌总体病人中,随T分期增加,CSS逐渐降低,HR逐渐升高。无淋巴结转移时,各组CSS和HR变化趋势与总体病人相同,各组病人CSS均较总体病人升高,其中T3期病人CSS上升明显,接近T2期病人。有淋巴结转移时,各组病人CSS均显著降低,T2期CSS最高,T1期和T2期病人组间HR在校正前(HR = 0.961,95% CI 0.599~1.539,P = 0.867)和校正后(HR = 0.886,95% CI 0.552~1.423,P = 0.616)差异均无统计学意义。结论:胃癌淋巴结转移对各组T分期显著降低生存率。无淋巴结转移时,T3期病人生存率升高,接近T2期病人。有淋巴结转移时,T1期生存率比T2期病人的预后更差。
Abstract:
Objective: To analyze the effect of lymph node metastasis on the specific survival rate of different T stages in gastric cancer. Methods: Based on the American SEER database, 1918 patients with gastric cancer with complete information from 2010 to 2015 were selected, and the specific survival rate (CSS) of gastric cancer was analyzed. Survival analysis was conducted using K-M method, univariate and multivariate Cox proportional hazard regression were used to evaluate the survival risk factors of independent prognosis, and the risk ratio of specific death (HR) was calculated and corrected 95% confidence interval (CI). The Bonferroni method was used to compare the groups one by one. Result: In total patients with gastric cancer, CSS decreased and HR increased with the increase of T stage. When there was no lymph node metastasis, the change trend of CSS and HR in each group was same as that in total patients, and the CSS in each group was higher than that in total patients, and the CSS in patients with stage T3 was significantly higher than that in patients with stage T2. When there was lymph node metastasis, the CSS of all patients decreased significantly, and the CSS of stage T2 was the highest. There was no significant difference in HR between stage T1 and stage T2 patients before (HR = 0.961, 95% CI 0.599 - 1.539, P = 0.867) and after adjustment (HR = 0.886, 95% CI 0.552 - 1.423, P = 0.616). Conclusion: Lymph node metastasis of gastric cancer significantly re-duced the survival rate of each T stage group. When there was no lymph node metastasis, the sur-vival rate of patients with stage T3 was higher, which was close to that of patients with stage T2. When there was lymph node metastasis, the survival rate of stage T1 was worse than that of stage T2.
1. 研究背景
胃癌是上消化系统第四常见的癌症,目前仍是全球第二大癌症相关死亡原因 [1] 。
根据胃癌的不同阶段,临床医生选择不同的治疗策略,如内镜治疗、胃切除术和全身治疗 [2] 。通常意义上认为在恶性肿瘤的发展过程中,肿瘤突变不断积累,在恶性肿瘤中,随着肿瘤浸润深度不断增加,预后逐渐变差。在最近一些有关其他肿瘤的研究中发现,某些肿瘤,如前列腺癌、结肠癌、乳腺癌 [3] ,在肿瘤早期即可有转移潜能出现,早期出现淋巴结转移的肿瘤甚至更低 [4] ,在胃癌中有关淋巴结转移对不同T分期特异性生存率研究仍较少。
SEER数据库(Surveillance, Epidemiology, and End Results)是世界上最大的癌症登记处之一,它是大规模的基于人群的癌症登记是确定癌症结果的有用工具 [5] 。通过系统地收集、存储和报告特定癌症患者的数据,癌症登记数据可以提供有见地的发现来指导临床实践。已被广泛用于多种肿瘤研究 [6] 。它具有病例资料覆盖广泛,病例数量巨大等优势 [7] 。本研究应用美国SEER数据库资料分析淋巴结转移情况对胃癌不同T分期胃癌特异性生存率的影响,为胃癌治疗策略选择提供证据支持。
2. 方法
2.1. 病例数据
数据来源于美国SEER数据库,使用SEER * Stat软件8.4.0.1版本,筛选了2010~2015年间接受根治术的胃癌病例。纳入标准:1) 胃癌原发灶的组织学诊断;2) 无胃切除术或其他恶性肿瘤病史;3) 确诊及手术时间为2010~2015年;4) 肿瘤TNM分期信息完整;5) 年龄18~84岁;6) 术后病理阴性切缘;7) 预后和随访信息完整;8) 术前无治疗。根据纳入和排除标准,确诊为胃癌病人共7065例,删除未知人种22例,剩余7034例,删除病理记录位置为胃食管连接处或转移癌病例2164例,剩余4879例,删除病理学分期记录为0 (36例)或NA (11例)的无效数据,剩余4832例,删除T分期记录为T0 (5例)和TX (737例),剩余4090例,删除N分期记录为NX (110例),剩余3980例,删除区域淋巴结总数记录不全数据102例3878例,删除阳性淋巴结数量记录不全数据1960例,最终剩余1918例病人进行分析(图1)。
2.2. SEER数据库筛选标准和结局
本文从SEER数据库中收集了以下病理和临床表现的可用信息:诊断时的年龄、性别、诊断年份、种族、原发部位、第7期AJCC分期信息、分级、淋巴结数据、具体死因死亡分类、生命状态和生存月份。主要研究结果是胃癌特异性生存期CSS。胃癌特异性生存期从诊断日期开始计算直至病人因胃癌死亡。死因分类为胃癌特异性或非胃癌相关死亡。其他原因的死亡或最后一次随访状态为存活的病例均记为存活,在统计分析时记为删失。由于SEER数据库的开源性质,本研究未获得知情同意。这项研究是根据《赫尔辛基宣言》进行的 [8] 。
2.3. 统计分析
使用SPSS Stata 14.0软件进行统计分析,生存概率采用Kaplan-Meier法计算,采用单因素及多因素Cox比例风险回归评估独立预后的生存危险因素,并以95%可信区间(CI)计算风险比(HR)。采用log-rank检验进行组间分析。应用似然比检验和Wald检验比较组间生存曲线,所有计算的P值均为两侧,P值小于0.05定义为有统计学意义。对于淋巴结阳性亚组和淋巴结阴性亚组的两两比较,采用n − 1的Bonferroni校正来调整多重比较,P值小于0.025定义为有统计学意义。
3. 结果
3.1. 病人特征
病人总数1918例,年龄为(62.63 ± 12.9)岁,≥50岁占84.9%,中位随访时间为64个月(IQR 54~73个月)。白种人占58.7%,黑种人占12.6%,其他人种主要为美国本土印第安人及环太平洋地区岛民,占比约28.7%。男性占60.2%,女性占39.8%;904例(47.1%) T分期比例分别为T1期438例(22.8%),T2期240例(12.50%),T3期706例(36.80%)。T4期534例(27.80%)。无淋巴结转移病人904例(47.1%),有淋巴结转移病人1014例(52.9%) (见表1和表2)。

Table 1. Comparison of T-stage groups of patients included
表1. 纳入病人的T分期分组比较

Table 2. Comparison of groups of patients with lymph node metastasis
表2. 纳入病人是否有淋巴结转移分组比较
按T分期比较,随着肿瘤侵犯深度的增加,中位随访时间减少,女性比例增加,整体年龄增加,淋巴结转移患者数量增加,远处转移数量增加(见表1)。
按淋巴结转移与否比较,淋巴结转移阳性患者的随访时间较短,白人比例较高,诊断年龄偏向老龄化,病理分期和T分期相对较高,远处转移比例较高(见表2)。
3.2. 生存分析
3.2.1. Kaplan-Meier曲线分析
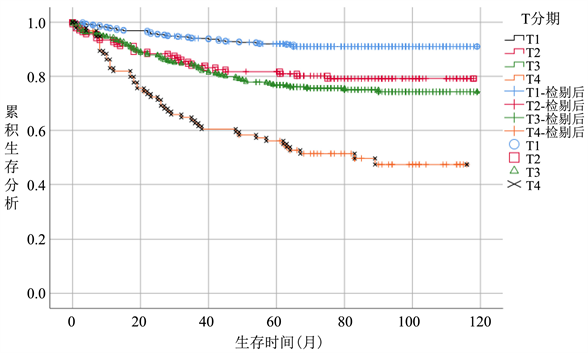
在整体病人中,随着T分期增加,病人CSS逐渐降低,T1期生存率最优,各个T分期间生存曲线差异明显。(图2(a))无淋巴结转移病人中,病人CSS曲线整体优于总体病人,其中T3期病人CSS升高明显,和T2期病人CSS较整体分析病人更为接近,差距变小。T4期病人生存曲线仍显著低于其他分期。(图2(b))而在有淋巴结转移的亚组病人中,T1期病人生存曲线下降明显,在早期低于T2期,接近T3期,随后部分随访时间段内稍优于T2,随访时间大于60个月以后始终低于T2期,除早期外,T3期始终低于T1、T2期,优于T4期生存概率(图2(c))。
 (a)
(a) 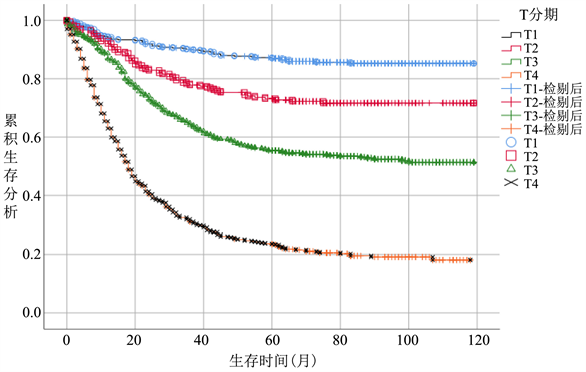 (b)
(b) 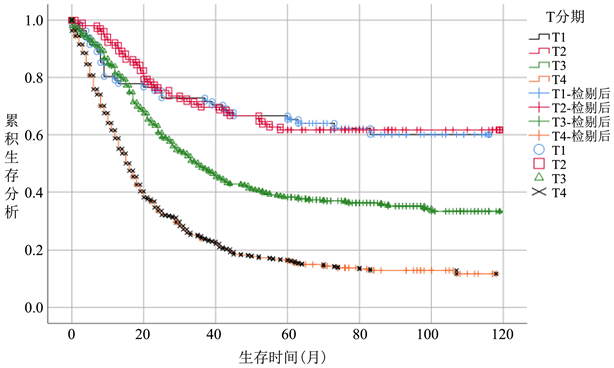 (c)
(c)
Figure 2. (a) Survival analysis T stage survival analysis of overall patient, (b) T stage survival analysis of patients without lymph node metastasis subgroup, (c) T stage survival analysis of patients without lymph node metastasis subgroup
图2. 生存分析(a) 总体病人T分期生存分析,(b) 无淋巴结转移亚组病人T分期生存分析,(c) 有淋巴结转移亚组病人T分期生存分析
3.2.2. COX分析
单因素Cox回归分析中,在整体分析中以T1N-组(HR = 1.00)为参照组,T4N-组(HR = 7.337,95% CI 4.661~11.549,P < 0.001)及T4N+ (HR = 22.054,95% CI 15.259~31.876,P < 0.001)的HR为各亚组最高;T2N+组(HR = 5.217,95% CI 3.255~8.362,P < 0.001) HR低于T1N+组(HR = 5.425,95% CI 3.297~8.925,P < 0.001) HR (见表3)。分层分析显示,无淋巴结转移病人中,与T1期相比,随着T分期增加,各组HR逐渐升高,分别为2.497,3.077,7.356,P值均<0.001;有淋巴结转移的病人中,与T1期(HR = 1.00)相比,T2期(HR = 0.961,95% CI 0.599~1.539,P = 0.867) HR降低,差异无统计学意义。T3、T4组HR分别为1.981,4.048,P值均<0.001。

Table 3. Comparison of HR values between different subgroups of TN stage
表3. TN分期不同亚组间HR值比较
在单因素COX分析发现,年龄分组(HR = 1.601;P < 0.001)、病理学分期(HR = 1.814;P < 0.001)、远处转移情况M分类(HR = 0.51;P < 0.001)是胃癌术后患者CSS的独立预测因子。3个因素在T分期预测胃癌CSS显著相关。所有3个特征均纳入多变量Cox比例风险模型(前向逐步过程),以校正队列中协变量的影响。其中年龄分组为18~30岁,31~40岁,41~50岁,51~60岁,61~70岁,71~80岁,81~84岁,性别分为男、女),病理学期分为1期、2期、3期、4期),M分期分为M0、M1,均为分类变量。多变量Cox分析结果显示,在整体分析中,以T1N-组(HR = 1.00)为参照组,T4N-组(HR = 6.864,95% CI 4.354~10.82,P < 0.001)及T4N+ (HR = 20.634,95% CI 14.221~29.939,P < 0.001)的HR较校正前稍低,仍为各亚组最高;在无淋巴结转移病人中,各组HR趋势与校正前相同,HR值相对校正前稍降低;在有淋巴结转移的病人中,T1期与T2期(HR = 0.886,95% CI 0.552~1.423,P = 0.616)之间HR差异无统计学意义(见表4)。

Table 4. Comparison of HR values among different subgroups of TN stages after correction
表4. 校正后TN分期不同亚组间HR值比较
注:校正因素包括年龄,远处转移情况,病理学分期。1) P < 0.05为差异有统计学意义。2) Bonferroni方法校正,P < 0.025为差异有统计学意义。
4. 讨论
在胃癌的分期中,应用最广泛的是美国癌症联合委员会(AJCC)肿瘤淋巴结转移(TNM)分期系统 [9] 。临床医生根据TNM分期决定治疗方案。从目前对胃癌的研究可知,多种预后因素影响胃癌患者的生存率,其中T分期对肿瘤大小对预后的影响有两个可能的原因。首先,当肿瘤侵犯粘膜下层以下时,对肿瘤大小的计算可能不准确。另一方面,当肿瘤局限于黏膜及黏膜下层时,肿瘤以水平生长为主,当肿瘤浸润内肌层时,以垂直生长为主 [10] 。在无淋巴结转移病人的生存分析中,T2 T3期病人生存曲线接近,这可能和肿瘤未突破浆膜,没有明显的播散有关。而在TNM分期中,各因素可能互相之间存在影响。有无淋巴结转移以及淋巴结数量被认为是多种癌症的独立预后因素 [11] 。淋巴结远处转移的风险随着浸润深度的加深也逐渐增加,即使在同一T分期,有无淋巴结转移患者预后明显不同,与本研究观察到的趋势一致。淋巴结转移对T分期影响预后的修饰效应在黑色素瘤中有相关报道 [4] ,但在胃癌研究中仍较少。在胃癌中,T1定义为肿瘤局限于粘膜或粘膜下层,T2定义为肿瘤浸润超过黏膜下层,但局限于固有肌层 [12] 。GC的自然病程不均匀,T1型GC进展缓慢 [13] ,但约20%的T1型GC患者发生淋巴结转移。由于临床特点和预后不同,T1期胃癌患者往往需要不同的治疗方法 [14] 。对于没有淋巴结转移的T1期胃癌患者,可采用内窥镜治疗。然而,内镜治疗有其适应症 [15] ,该技术不能解决转移性淋巴结的问题,针对有淋巴结转移的病人,应接受根治性手术切除和辅助化疗,合并远处转移的需要接受全身治疗,如姑息性化疗、分子靶向抗肿瘤药物和免疫治疗 [16] 。
本研究对整体和无转移淋巴结患者进行分析,在校正混杂因素前后均有相似的结果和趋势,随着T分期的增加,胃癌特异性生存的HR逐渐升高且预后逐渐恶化,P值均<0.025,各组间差异有统计学意义;在有转移淋巴结的亚组分析中,校正前T2分期的患者预后最好,与T1分期和T2分期相比,HR = 0.961,P = 0.867差异无统计学意义。校正可能的混杂因素后,淋巴结阳性患者中,T1和T2分期HR = 0.886,P = 0.616,差异仍无统计学意义。也就是说,在有淋巴结转移时,T2期相对于T1期病人,对CSS的影响无统计学意义。T分期对胃癌生存的影响在有无淋巴结转移时存在显著差异。在本研究中,在淋巴结阳性患者亚组中,各个T分期CSS均显著降低,尤其T1期CSS降低至并不优于T2期,即较低T期发生淋巴结转移可能代表肿瘤恶性程度较高。相反,较大的T分期仍无淋巴结转移可能意味着低转移潜能的肿瘤应该有相对较好的预后 [17] 。提示临床医生针对胃癌患者进行术前评估时,尤其是T1期准备接受内镜治疗的胃癌患者,不仅要重视浸润深度的判断,更要加强区域淋巴结的术前影像学检查,术后也要加强随访,复查淋巴结情况。
本研究应用的SEER数据库是美国权威的癌症统计数据库,它是北美最具代表性的大型肿瘤登记注册数据库之一,它的大规模数据优势是无可比拟的 [18] 。但是不可避免的,SEER数据库中可能出现编码错误和分类错误 [19] 。同时这种回顾性分析缺乏淋巴结转移及远处转移的具体部位和一些关键的危险因素如HP感染,关键分子如Her-2的检测,以及缺少围手术期化疗、术后化疗等重要的治疗信息。此外,亚洲和西方癌症中心对同一TNM类别的局部晚期GC的治疗方案不同,西方国家通常采用新辅助治疗 + 根治性切除(包括D1或D1 + 淋巴结清扫),而亚洲癌症中心主要考虑根治性手术(D2或D2 + 淋巴结清扫) + 辅助治疗 [20] 。希望本文能为中国胃癌治疗策略选择提供参考,未来仍需更多数据进一步验证。
NOTES
*通讯作者。