1. 引言
光催化分解水是解决全球能源短缺和环境污染问题的有效手段,可以将太阳能转化为清洁和可再生的氢气。金属氮化物具有比它们氧化物更窄的带隙,可见光响应范围更大,催化性能更高而备受关注。钽基氮化物因其良好的电热稳定性、合适的带隙、储量丰富、无毒等特点,光催化应用前景广阔 [1] [2] [3] 。传统的Ta3N5基光催化材料颗粒较大,比表面积小、输运性差、光生载流子复合快、表面催化反应动力学慢,上述缺点严重限制了其光催化应用 [4] 。形貌控制工程可以提高材料的光催化活性和选择性,不同暴露表面表现出不同的催化活性 [5] [6] 。Ta3N5基光催化材料形貌控制研究对于提升其催化性能极其必要。
研究表明,通过不同合成路线将钽基氮化物设计成纳米颗粒、纳米片、纳米球、纳米网、纳米线和纳米管等形貌,其催化活性得到明显提升 [7] [8] 。控制合成特殊纳米结构的Ta3N5,如Cui等 [9] 以HF、HCl、H2O2为形貌控制剂利用水热合成以及高温氮化技术制得了Ta3N5 3D纳米花状层级结构,可以提供更多活性位点,改善光生载流子的输运性能和缩短光生载流子传输距离,进而提高催化活性 [10] [11] 。前期,课题组构建了Ta2O5@TaON@Ta3N5 n-n突变异质结构,驱动光生载流子由内向外定向快速迁移至样品表面,促进光生载流子高效分离,显著提升样品光催化产氢性能 [12] 。
本文采用水热合成与高温氮化联合技术,建立了核壳结构Ta2O5@ Ta3N5纳米花控制合成工艺,为进一步开发新型高活性Ta3N5基纳米光催化材料的奠定实验技术基础。
2. 实验部分
2.1. 仪器与试剂
试剂:五氯化钽(分析纯),湖南省华京粉体有限公司;盐酸(分析纯),西陇化工股份有限公司;氢氟酸,(分析纯),天津市科密欧化学试剂有限公司;乙醇(分析纯),天津市光复科技有限公司;异丙醇(分析纯),天津市富宇精细化工有限公司。
仪器:热场发射电子扫描电镜SU-70,日本日立公司;X射线衍射仪XRD-6000,日本岛津公司;电热风鼓干燥箱DHG-9030A,天津市泰斯特仪器有限公司;透射电子显微镜Tecnai G2TF20,美国FEI公司;管式炉MXG1200-40S,上海微行炉业有限公司。
2.2. Ta2O5@Ta3N5纳米花制备
准确称取0.2687 g TaCl5分别于盛有14 mL的异丙醇溶液(水、乙醇溶液、乙醇和异丙醇溶液)的烧杯中,在室温条件下,边搅拌边加入4.8 mL体积的蒸馏水,再加入浓HF 200 µL溶液和浓HCl溶液400 µL (800 µL、600 µL、200 µL)。充分续搅30 min后,确保所有反应试剂能够均匀分散,将所得澄清混合液转移到聚四氟乙烯反应釜中,经160℃水热4 h (3 h)后,冷却至室温,洗涤过滤烘干,750℃焙烧2 h将所得产物平铺在刚玉瓷舟中,放置于管式炉中通入氨气,经850℃氮化3 h,制得核壳结构Ta2O5@Ta3N5纳米花。
3. 结果与讨论
3.1. Ta2O5纳米花的可控制备
3.1.1. 溶剂种类对Ta2O5纳米花形貌的影响
图1是以不同溶剂经过160℃水热3 h所制备出的Ta2O5纳米催化剂的SEM图。可见,溶剂对Ta2O5形貌有明显影响,溶剂中醇用量增加,样品生长出更多的纳米棒。图1(a)是以水为溶剂制备样品呈无规则形貌的团聚体。图1(b)是以乙醇溶液为溶剂制备样品呈大小不均匀的圆片。图1(c)是以异丙醇溶液为溶剂制备样品呈由纳米棒(长约200 nm、直径约30~50 nm)构成的花状形貌。图1(d)是以乙醇和异丙醇溶液为溶剂制备样品呈球型,表面出现了少量的棒状结构。异丙醇作为反应介质,可以控制样品花状层级结构的生长 [13] [14] ,还能增强Ta2O5表面酸根离子吸附的稳定效应,促进其择优生长。因而,选择异丙醇为溶剂进行后续研究。
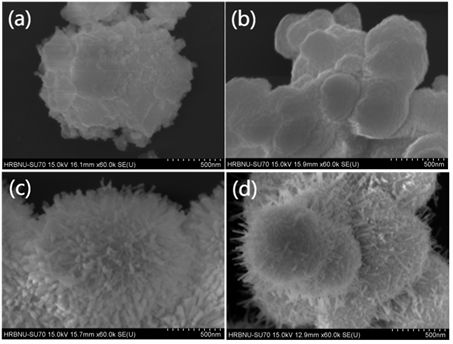
Figure 1. SEM images of Ta2O5 nanoflowers prepared using (a) water (b) ethanol (c) isopropyl alcohol (d) isopropyl alcohol, and ethanol as solvents
图1. 分别以(a) 水、(b) 乙醇、(c) 异丙醇、(d) 异丙醇和乙醇为溶剂制得Ta2O5纳米花的SEM图
3.1.2. 异丙醇用量对Ta2O5纳米花形貌的影响
调控异丙醇用量对Ta2O5形貌的影响如图2所示。可见,随着异丙醇含量升高,纳米棒状结构增多,纳米棒变细,棱角清晰。图2(a)异丙醇用量为8 mL,明显存在较多的无规则形貌团聚体,纳米棒状结构较少。图2(b)异丙醇用量增加到10 mL,出现较为明显的花状层级结构。图2(c)异丙醇用量为12 mL,纳米棒较均匀放射状排布,花状层级结构更明显。图2(d)异丙醇用量为14 mL,纳米棒状结构更多,长约200 nm,呈放射状均匀排布,形成良好的纳米花状层级结构。可见,异丙醇用量对Ta2O5形貌调控作用明显,异丙醇含量增多,异丙醇与Ta2O5表面酸根离子吸附的稳定性越大,控制其择优生长,确定异丙醇最佳用量为14 mL。
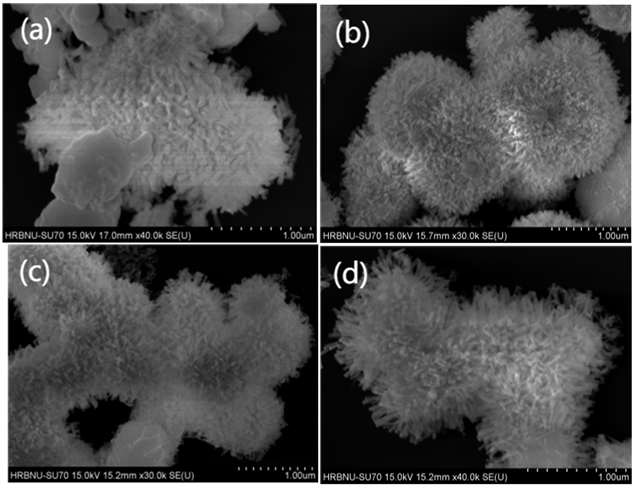
Figure 2. SEM images of Ta2O5 nanoflowers prepared with (a) 8 ml (b) 10 ml (c) 12 ml (d) 14 ml isopropyl
图2. 异丙醇用量为(a) 8 ml、(b) 10 ml、(c) 12 ml、(d) 14 ml所制备Ta2O5纳米花的SEM图
3.1.3. 盐酸用量对Ta2O5纳米花形貌的影响
图3为调控盐酸用量制备Ta2O5纳米光催化剂的SEM图像。显然,随着盐酸用量从800 µL减小到400 µL,Ta2O5纳米花的棒状增多,花状结构层级明显,说明酸量较大抑制Ta2O5纳米棒生长。当盐酸用量从400 µL继续减小到200 µL时,棒状结构减少。因此,盐酸最佳用量为400 µL。
3.1.4. 水热时间对Ta2O5纳米花形貌的影响
为了获得更具优势形貌的Ta2O5前驱体,利用上述实验确定的优化工艺条件,调控水热时间对Ta2O5形貌的影响如图4所示。可见,水热时间从3 h增加到4 h,Ta2O5纳米花的棒状结构增多,纳米棒变长,分布均匀分散,花状形貌完整,说明充分水热时间是保障纳米棒完全生长的关键因素。因此,确定最佳水热时间为4 h。

Figure 3. SEM images of Ta2O5 nanoflowers prepared with (a) 800 µL, (b) 600 µL, (c) 400 µL, (d) 200 µL HCl amounts, respectively
图3. HCl用量为(a) 800 µL、(b) 600 µL、(c) 400 µL、(d) 200 µL所制备Ta2O5纳米光花的SEM图像

Figure 4. SEM images of Ta2O5 nanoflowers prepared with a hydrothermal time of (a) 3 h, (b) 4 h
图4. 水热时间为(a) 3 h、(b) 4 h所制备Ta2O5纳米花的SEM图
3.2. Ta2O5@Ta3N5纳米花高温氮化工艺
图5为经850℃高温氮化3 h制备Ta2O5@Ta3N5纳米催化剂的SEM图像。可见,经过高温氮化,样品形貌略有改变,纳米棒出现局部晶格塌陷,晶体棒状结构略有弯曲。这是由于高温氮化过程中,Ta2O5经拓扑转化生成Ta2O5@Ta3N5,其结构框架未发生明显改变,仍保持Ta2O5的花状层级结构,2个N原子替代3个O原子形成氧空位而引起局部晶格塌陷,致使棒状结构弯曲。

Figure 5. SEM images of (a) Ta2O5 and (b) Ta2O5@Ta3N5nanoflowers
图5. (a) Ta2O5和(b) Ta2O5@Ta3N5纳米花的SEM图
3.3. Ta2O5@Ta3N5纳米花的表征
3.3.1. XRD分析
图6为样品XRD谱。由图6可见,位于17.20˚、24.46˚、31.36˚、34.90˚、35.93˚、39.26˚、44.10˚处的特征衍射峰对应于Ta3N5 (002)、(110)、(023)、(004)、(113)、(024)和(043)晶面(JCPDS No. 79-1533) [12] ;位于30.0˚、47.58˚处较弱的衍射峰归属于Ta2O5的(112)和(123)晶面(JCPDSNo.54-0432) [15] ,源于样品未氮化的Ta2O5核芯。

Figure 6. XRD pattern of Ta2O5@Ta3N5 sample
图6. Ta2O5@Ta3N5样品的XRD谱
3.3.2. HRTEM和BET分析
如图7(a)为Ta2O5@Ta3N5的HRTEM图。可见,晶格间距为0.363 nm的衍射条纹归属于Ta3N5 (110)晶面 [10] ,晶格间距为0.368 nm的衍射条纹对应于Ta2O5 (211)晶面 [13] ,进一步证实Ta2O5@Ta3N5的核壳异质结构。图7(b)为Ta2O5@Ta3N5的N2吸附–脱附等温曲线,显示该样品为IV型等温线,在相对压力范围内(0.2~0.95)具有H3型滞后环,BET比表面积为21.9 m2∙g−1。图7(c)为孔径分布图,表明该样品存在狭缝状介孔结构,以平均孔径为24 nm的介孔为主,由棒状结构堆积而成,有利于光催化反应传质过程,进而提升催化性能。


Figure 7. (a) HRTEM image of Ta2O5@Ta3N5 sample, (b) N2 adsorption-desorption isotherms, (c) pore size distributions
图7. (a) Ta2O5@Ta3N5样品的HRTEM图 (b) N2吸附–脱附等温线 (c) 孔径分布
4. 结论
本文利用水热合成和高温氮化技术成功控制合成了核壳结构的Ta2O5@Ta3N5纳米花。控制合成前驱体Ta2O5纳米花的优化工艺条件为:选用异丙醇溶液为溶剂,异丙醇用量为14 mL,浓盐酸用量为400 µL,经160℃水热时间为4 h。然后,在50 mL∙min−1 NH3气流下,经850℃高温氮化3 h,Ta2O5纳米花拓扑转化层级结构Ta2O5@Ta3N5纳米花,纳米棒长约200 nm,直径约50 nm,比表面积为21.9 m2∙g−1。