1. 引言
众所周知,全髋关节置换术(THA)和全膝关节置换术(TKA)是治疗骨关节炎的有效方法,然而,术后深静脉血栓(DVT)仍然是THA、TKA术后最严重的并发症之一 [1] 。研究表明,未接受药物治疗的患者有40%~60%的深静脉血栓发生率和1%~2%的肺栓塞(PE)发生率 [2] 。而深静脉血栓和肺栓塞不仅降低患者术后满意度、增加住院费用,甚至可能威胁到患者生命 [3] 。因此THA和TKA术后静脉血栓的预防至关重要。
预防静脉血栓栓塞的抗凝剂包括简单的口服药物(阿司匹林)、注射药物(低分子肝素)和新型口服药物(利伐沙班和达比加群酯)等 [4] 。阿司匹林作为预防血栓药物具有价格低廉,易于服用,不需要血液监测,耐受性好,安全性高等特点 [3] [4] [5] [6] [7] 。2012年,ACCP批准阿司匹林用于TKA和THA术后静脉血栓栓塞预防,1B级(中度证据),与无预防措施相比,注射低分子肝素和新型的口服药物的证据水平相同 [4] 。2018年,NICE推荐阿司匹林单独作为TKA后静脉血栓栓塞预防的一种选择;然而,THA术后的患者在服用阿司匹林前需要使用10天的低分子肝素,或者单独服用更新、更昂贵的新型口服药物或低分子肝素 [8] 。随着阿司匹林在ACCP等许多国际指南中得到认可,以及更多新出现的证据证明其疗效,阿司匹林作为TKA和THA手术之后的VTE预防药物在骨科界开始受到欢迎 [6] [9] [10] 。
因此阿司匹林在TKA和THA术后作为抗凝药物的使用需要高质量、大样本的随机对照试验(RCT)作为依据。故本研究通过系统的收集国内外关于阿司匹林在关节置换术后作为血栓预防药物的随机对照试验(RCT),采用Meta分析和系统回顾的方法综合分析纳入的临床研究,以期在TKA和THA术后抗凝药物的选择上给广大关节外科医生提供可靠的循证依据。
2. 资料与方法
(一) 文献检索
计算机检索PubMed,MEDLINE,Embase,Web of Science,Cochrane Library databases等外文数据库(包括但不限于英语),中国知网、万方、维普等中文数据库建库至2022年5月28日以来的所有相关研究。英文检索词包括:人口学(Population)关键词(knee arthroplasty,hip arthroplasty,knee replacement,hip replacement等及其Mesh词),干预(Intervention)关键词(prophylaxis,aspirin等及其Mesh词),对照(Comparison)关键词:(heparin, low molecular weight heparin, dabigatran, rivaroxaban, warfarin),结果(Outcome)关键词(deep vein thrombosis,venous thromboembolism,pulmonary embolism,bleeding等及其Mesh词)。中文检索词包括:全膝关节置换术、全髋关节置换术、关节置换术、阿司匹林、乙酰水杨酸、肝素、华法林、达比加群、利伐沙班、阿哌沙班、静脉血栓、肺栓塞、出血等。
(二) 纳入标准与排除标准
纳入标准:1) 自建库以来至2022年4月间,国内外已公开发表的文献;2) 研究类型为随机对照试验(RCT);3) 研究对象为年满18周岁的、手术方式为TKA或THA的患者;4) 研究对象随机分为阿司匹林组和对照组(不包括空白对照);5) 研究主要结果为深静脉血栓形成、肺栓塞、出血等相关并发症,次要结果为伤口感染、假体周围感染等并发症;
排除标准:1) 非随机对照试验;2) 对照组为空白对照的随机对照试验;3) 随访时间少于2周;4) 已完成注册但尚未出版或尚未完成的临床研究;
(三) 数据提取与质量评价
数据提取:严格按照纳入标准筛选相关研究,提取作者信息、发表年份、国家、纳入样本量、人口学分布情况、分组情况以及相关结果。
文献质量评价:根据Cochrane干预系统评价指南 [11] Cochrane偏倚风险评估工具评价随机对照试验偏倚风险(分为低风险、不明风险、高风险)。
数据提取有两名研究者独立完成,如有争议之处,由第三名研究者来综合判定。文章的检索以及筛选严格按照预先制定的纳入和排除标准执行。在不同的数据库之间重复的研究中,纳入最新、最全面的研究。
(四) 数据分析
1) 合并效应指标
采用Review Manager 5.4.1软件(Cochrane Collaboration, Software Update, Oxford, United Kingdom)进行数据统计分析。对于二分类变量数据统计描述采用比值比(OR)及95%可信区间(CI),对于连续变量数据统计描述采用均数差(MD)及95%可信区间(CI)。文献异质性采用c2检验,如果R ≥ 0.1且I2 ≤ 50%,我们认为纳入的文献之间异质性较小,故采用固定效应模型(Fixed Effects Model);如果R ≤ 0.1且I2 ≥ 50%,我们认为纳入的文献之间异质性较大,采用随机效应模型(Random Effects Model)。所有统计结果以森林图的形式展示。
2) 亚组分析
为了避免各个研究特征对于总体效应指标产生较大的影响,归纳总结研究特征并对其进行亚组分类分析,如果组间异质性较大,可能会对总效应指标产生影响,则分析原因。亚组分析的研究特征包括:研究地区,手术类型,是否使用物理预防血栓,血栓诊断方法及比较的药物类型。
3) 发表偏倚
仅针对TKA及THA术后深静脉血栓发生率进行发表偏倚分析,结果通过漏斗图的形式呈现。
3. 结果
(一) 研究纳入结果及质量评价
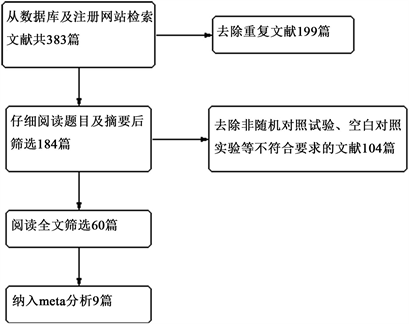
Figure 1. Literature selection flow chart, 9 literatures were finally included
图1. 文献筛选流程图,最终纳入9篇文献
一共检索出相关文献383篇,重复文献199篇,去除不符合纳入标准的非随机对照试验及空白对照试验104篇,最终全文阅读潜在相关的文献60篇,共筛选出9篇符合纳入标准的较高质量的随机对照试验。筛选纳入流程见图1。纳入的研究共包括5591例患者,2775例阿司匹林组患者和2816例对照组患者,其中1915例为男性,2001例为女性。对照组包括1813例利伐沙班组患者,低分子肝素组867例,华法林组146例,低分子右旋糖酐组50例。所有纳入的患者中2830例为全膝关节置换术(TKA)组,2761例为全髋关节置换术(THA)组(研究特征总结见表1)。
纳入研究的偏倚风险评估结果如图2所示。几乎所有的研究均包括至少一项未知风险的偏倚风险。有1篇研究的分配方法隐藏偏倚风险为高风险。
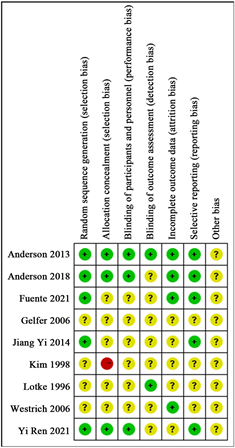
Figure 2. Included study bias risk assessment
图2. 纳入研究偏倚风险评估

Table 1. Basic characteristics of 9 literature studies included
表1. 纳入9篇文献研究基本特征
(二) Meta分析结果
1) 术后深静脉血栓发生率
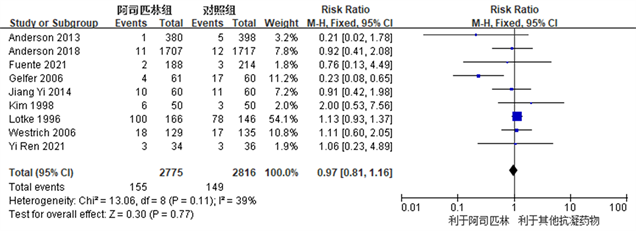
Figure 3. Forest plot comparing the incidence of deep vein thrombosis after joint replacement surgery between the aspirin group and the control group
图3. 阿司匹林组与对照组关节置换术后深静脉血栓发生率比较的森林图
各个研究都有术后深静脉血栓发生,阿司匹林组和对照组之间深静脉血栓发生率未见明显统计学差异(RR: 0.90, 95% CI: 0.63~1.29, I2 = 39%, R = 0.57) (图3所示)。因R ≥ 0.1且I2 ≤ 50%,我们认为各项研究之间异质性较小,故采用固定效应模型。结果提示,阿司匹林在预防TKA及THA术后深静脉血栓发生方面疗效与临床常用抗凝药物相同。
2) 术后肺栓塞发生率

Figure 4. Forest chart comparing the incidence of pulmonary embolism after joint replacement surgery between the aspirin group and other anticoagulant drug groups
图4. 阿司匹林组与其他抗凝药物组关节置换术后肺栓塞发生率比较的森林图
共有7项研究 [3] [7] [12] [13] [14] [17] [18] 观察并比较了阿司匹林组和对照组之间肺栓塞的发生率,两个组之间肺栓塞发生率未见明显统计学差异(RR: 0.91, 95% CI: 0.53~1.56, I2 = 0%, R = 0.72) (图4所示)。因R ≥ 0.1且I2 ≤ 50%,我们认为各项研究之间异质性较小,故采用固定效应模型。结果提示,阿司匹林在TKA及THA术后预防肺栓塞发生方面疗效与临床常用抗凝药物相同。
3) 出血事件发生率
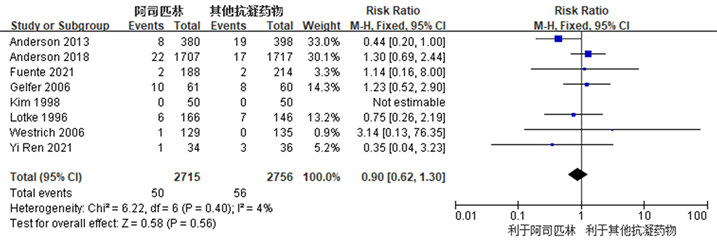
Figure 5. Forest chart comparing the incidence of bleeding events after joint replacement surgery between the aspirin group and the control group
图5. 阿司匹林组与对照组关节置换术后出血事件发生率比较的森林图
共有8项研究 [3] [7] [12] [13] [14] [15] [17] [18] 观察比较了阿司匹林组和对照组之间术后出血事件发生率,两个组之间术后出血事件发生率未见明显统计学差异(RR: 0.90, 95% CI: 0.62~1.30, I2 = 4%, R = 0.40) (图5所示)。因R ≥ 0.1且I2 ≤ 50%,我们认为各项研究之间异质性较小,故采用固定效应模型。结果提示,阿司匹林在TKA及THA术后作为预防血栓抗凝药物,具有与常用抗凝药物同样的出血事件发生率。
4) 伤口并发症发生率
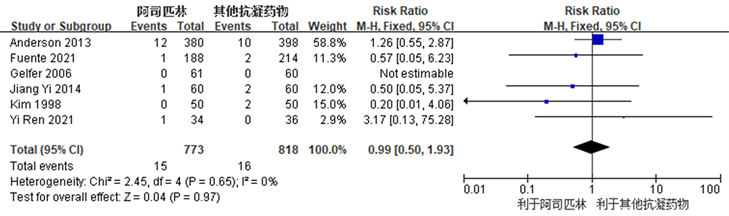
Figure 6. Forest chart comparing the incidence of wound complications after joint replacement surgery between the aspirin group and the control group
图6. 阿司匹林组与对照组关节置换术后伤口并发症发生率比较的森林图
共有6篇研究 [3] [12] [13] [15] [16] [17] 观察比较了阿司匹林组和对照组之间术后伤口并发症发生率,两个组之间术后伤口并发症发生率未见明显统计学差异(RR: 0.99, 95% CI: 0.50~1.93, I2 = 0%, R = 0.65) (图6所示)。因R ≥ 0.1且I2 ≤ 50%,我们认为各项研究之间异质性较小,故采用固定效应模型。结果提示,阿司匹林在TKA及THA术后作为预防血栓抗凝药物,具有与常用抗凝药物同样的伤口并发症发生率。
5) 胃肠道不良反应发生率
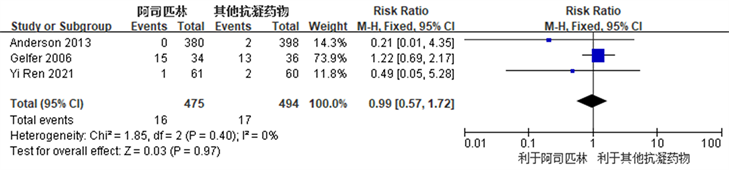
Figure 7. Forest plot comparing the incidence of gastrointestinal adverse reactions after joint replacement surgery between the aspirin group and the control group
图7. 阿司匹林组与对照组关节置换术后胃肠道不良反应发生率比较的森林图
共有3项研究 [3] [12] [17] 观察比较了阿司匹林组和对照组之间术后胃肠道不良反应发生率,两个组之间术后胃肠道不良反应发生率未见明显统计学差异(RR: 0.99, 95% CI: 0.57~1.72, I2 = 0%, R = 0.40) (图7所示)。因R ≥ 0.1且I2 ≤5 0%,我们认为各项研究之间异质性较小,故采用固定效应模型。结果提示,阿司匹林在TKA及THA术后作为预防血栓抗凝药物,具有与常用抗凝药物同样的胃肠道不良反应发生率。
6) 围术期总出血量
共有5项研究 [3] [7] [12] [14] [15] 观察比较了阿司匹林组和对照组之间围术期总出血量,异质性检验提示研究间存在较大异质性(I2 = 59%, R = 0.04),采用敏感性分析排除可能存在较大异质性的研究 [14] 后,无统计学异质性(I2 = 23%, R = 0.27),采用固定效应模型。结果提示,阿司匹林在TKA及THA术后作为预防血栓抗凝药物,术后总出血量方面与常用抗凝药物术后总出血量相同(MD: −6.76, 95% CI: −31.41~17.89, I2 = 23%, R = 0.59) (图8所示)。
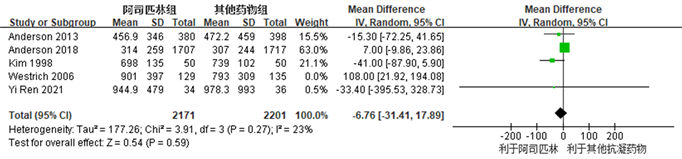
Figure 8. Forest map of the comparison of total perioperative bleeding between the aspirin group and the control group after joint replacement surgery
图8. 阿司匹林组与对照组关节置换术后围术期总出血量比较的森林图
(三) 亚组分析
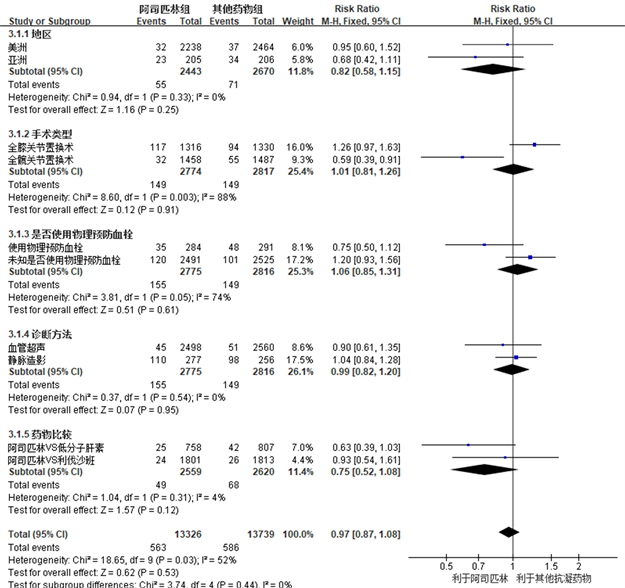
Figure 9. Shows a forest map of subgroup analysis comparing the incidence of deep vein thrombosis after joint replacement surgery between the aspirin group and other anticoagulant drug groups based on the study characteristics included in the analysis: study area, surgical type, whether physical prevention of thrombosis is used, diagnostic methods for thrombosis, and types of drugs compared
图9. 根据纳入分析的研究特征:研究地区、手术类型、是否使用物理预防血栓、血栓诊断方法及比较的药物类型等,对阿司匹林组与其他抗凝药物组关节置换术后深静脉血栓发生率进行亚组分析比较的森林图
我们以TKA及THA术后深静脉血栓发生率作为效应指标,根据研究特征进行了亚组meta分析,纳入分析的研究特征包括:研究地区,手术类型,是否使用物理预防血栓,血栓诊断方法及比较的药物类型。分析结果提示上述的研究特征对于效应指标没有明显的影响(RR: 0.97, 95% CI: 0.87~1.08)。在手术类型和是否使用物理预防血栓亚组分析中,虽然总体效应提示无明显统计学差异(RR: 1.01, 95% CI: 0.81~1.26, R = 0.91) (RR: 1.06, 95% CI: 0.85~1.31, R = 0.61),但是组间异质性分析提示全膝关节置换术和全髋关节置换术(c2 = 8.60, I2=88%, R = 0.003)、使用物理预防血栓和未使用/未知使用物理预防血栓(c2 = 3.81, I2 = 74%, R = 0.05)术后深静脉血栓发生率存在着差异。在比较阿司匹林VS利伐沙班 [3] [7] [16] 和阿司匹林VS低分子肝素 [12] [13] [14] [17] 的研究之间,术后深静脉血栓发生率未见明显差异(RR: 0.75, 95% CI: 0.52~1.08, R = 0.12)。在美洲进行的研究 [7] [12] [13] [14] [18] 和亚洲进行的研究 [3] [15] [16] [17] 之间,术后深静脉血栓发生率同样未见明显差异(RR: 0.82, 95% CI: 0.58~1.15, R = 0.25)。在深静脉血栓诊断方法为血管超声 [3] [7] [12] [13] [14] [16] 和静脉造影 [15] [17] [18] 的研究之间,术后深静脉血栓发生率未见明显差异(RR: 0.99, 95% CI: 0.82~1.20, R = 0.95) (图9所示)。
(四) 发表偏倚
发表偏倚仅针对TKA及THA术后深静脉血栓发生率进行,本研究共纳入9篇随机对照试验进行分析,漏斗图结果显示一个研究未在95% CI线之内,提示可能存在发表偏倚,但纳入的研究总体分布对称,因而可以不考虑发表偏倚引起的影响(图10所示)。

Figure 10. Publication bias funnel chart of 9 articles included in the evaluation of effectiveness and safety in preventing deep vein thrombosis after joint replacement surgery
图10. 纳入的9篇文献进行关节置换术后深静脉血栓预防的有效性及安全性评估的发表偏倚漏斗图
4. 讨论
目前TKA及THA术后预防深静脉血栓药物的选择仍然存在争议 [6] [10] [19] [20] 。近年来在北美,关节置换术后VTE预防的药物方案在向使用阿司匹林在转变 [10] 。最近的研究也报告了阿司匹林在预防术后下肢静脉血栓形成和肺栓塞方面的疗效 [19] [20] [21] [22] 。多项研究表明在用于膝关节和髋关节置换术后预防血栓形成方面,与利伐沙班、依诺肝素相比,阿司匹林显著降低了出血并发症的几率 [9] [23] [24] 。 而Sørensen等 [25] 对5824名静脉血栓栓塞患者和58240名正常人群进行的病例对照研究表明,阿司匹林对预防静脉血栓栓塞没有预防作用。
因此我们的meta分析以TKA及THA术后在抗凝药物的选择上,对比阿司匹林和其他常见抗凝药物(利伐沙班,低分子肝素等),比较术后深静脉血栓等并发症的发生率及药物相关出血风险。共纳入9篇RCT [3] [7] [12] - [18] 进行meta分析。与既往的meta分析 [4] [19] [26] [27] [28] 不同,本研究新增2个最新、较高质量的RCT [3] [13] ,可能会对最终结果产生改变。结果提示,阿司匹林在预防TKA及THA术后深静脉血栓发生率、肺栓塞发生率、出血事件发生率、伤口并发症发生率、胃肠道不良反应发生率等方面与常见抗凝药物(低分子肝素,利伐沙班等)没有明显的统计学差异。本研究结论与既往其他研究 [4] [19] [26] [27] [28] 结论一致。
从分子机制上讲,阿司匹林的抗血小板作用是通过不可逆抑制血小板环氧合酶-1 (COX-1),从而阻止血栓素A2 (TXA2)以及其非活性代谢物血栓素B2 (TXB2)的合成。而血栓素A2是一种有效的、短期的血小板激动剂和血管收缩剂 [29] 。然而,COX-1在胃肠道粘膜等其他组织中也表达,对胃肠道黏膜的保护具有重要的作用 [30] 。故长期服用阿司匹林可能会影响胃肠道黏膜的修复作用,引起胃肠道不良反应。在最近的一项调查中,15%接受低剂量阿司匹林治疗的患者出现上消化道症状 [31] 。我们的研究结论显示,阿司匹林与其他抗凝药物在胃肠道不良反应方面未见明显差异,进一步表示阿司匹林的药物安全性与其他常用抗凝药物相同。
另外,国外研究报道,30天服用利伐沙班的成本约为379美元至450美元 [32] [33] 。使用低分子肝素的大致成本约450美元至890美元 [33] [34] 。与之相比,阿司匹林口服一个月仅需要2美元,而且不需要常规血液检测 [35] 。阿司匹林在药物费用方面的优势能为患者减少医疗费用、减轻负担,能为国家节省不少的医疗资源投入。
有研究证明与325 mg阿司匹林相比,81 mg阿司匹林可显著减少胃肠道不适和恶心等不良反应 [10] [36] [37] 。故纳入本研究的绝大多数研究 [3] [7] [12] [13] [15] [16] [17] 都使用了100 mg左右量/天的阿司匹林作为标准进行了试验,而相对较早的一些研究 [14] [18] 使用了325 mg/天量的阿司匹林作为标准,而后者可能在一定程度上增加了这些研究中阿司匹林引起的出血不良反应、胃肠道不良反应的风险。在进行围术期总出血量异质性检查时发现研究之间异质性明显,故排除采用敏感性分析排除可能存在较大异质性的研究 [14] 后,无统计学异质性,进一步证实了上述观点。组间异质性分析提示全膝关节置换术VS全髋关节置换术、使用物理预防血栓VS未使用/未知使用物理预防血栓组术后深静脉血栓发生率存在着统计学差异,提示全膝关节置换术和未使用使用物理预防血栓的患者术后相对更容易发生术后深静脉血栓形成。
本研究存在以下几个缺点:第一,纳入研究数量仅为9篇,按照入选标准排除较多的低质量研究,纳入数量较少可能会导致论证强度不足;第二,纳入的研究在研究地区,手术类型,是否使用物理预防血栓,血栓诊断方法及比较的药物类型等方面存在着差异,虽然亚组分析提示没有明显异质性,以及进行敏感性分析排除存在较大异质性的研究,但是仍然可能会导致偏差;第三,几乎所有的研究均包括至少一项未知风险的偏倚风险 [3] [7] [12] - [18] ,有1篇研究的分配方法隐藏偏倚风险为高风险 [15] 。
综上所述,我们认为阿司匹林与临床常用抗凝药物在疗效和安全性方面无明显差异,可单独使用于全膝关节置换术、全髋关节置换术后预防深静脉血栓形成。结论需要更多高质量随机对照试验(RCT)来提供更多可靠依据、加强可靠性。