摘要: 细支气管腺瘤(bronchiolar adenoma, BA)是WHO (2021)胸部肿瘤中新定义的一种临床表现惰性的较为少见的实体肺肿瘤,且易与黏液腺癌相混淆,尤其在术中冰冻时。本文报道2例女性病例,分别发生于右肺下叶和左肺上叶,镜下表现为肿瘤组织呈腺样排列,主要由纤毛细胞、黏液细胞、立方细胞构成,基底细胞部分缺失。免疫表型:肿瘤细胞CK7、TTF-1、NapsinA均阳性,基底细胞p40、p63、CK5/6阳性(例2仅p63强弱不等),p53呈野生型表达,EGFR阳性,Ki-67阳性指数分别约1%和10%,例2 ALK (D5F3)阳性、PD-L1 (SP263) TPS值 < 1%。通过本文报道的2例BA的复习,了解其病理形态及免疫表型特征,避免误诊。
Abstract:
Bronchiolar adenoma (BA) is a relatively rare solid lung tumor newly defined by WHO (2021) in the field of chest tumors, with inert clinical manifestations, and is easily confused with mucinous ade-nocarcinoma, especially during intraoperative freezing. This article reports two female cases, occur-ring, respectively in the right lower lobe and left upper lobe. Microscopically, the tumor tissue ap-pears as glandular arrangement, mainly composed of ciliated cells, mucus cells, and cuboid cells, with partial absence of basal cells. Immunophenotypes: tumor cells CK7, TTF-1, and NapsinA are all positive, while basal cells p40, p63, and CK5/6 are positive (case 2 only shows varying strength of p63). p53 is expressed in the wild-type, EGFR is positive, and Ki-67 positivity index is about 1% and 10%. ALK (D5F3) of Case 2 is positive, and PD-L1 (SP263) TPS value is less than 1%. By reviewing the two cases of BA reported in this article, we aim to understand their pathological morphology and immunophenotypic characteristics to avoid misdiagnosis.
1. 病例报告
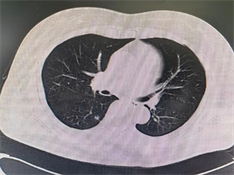
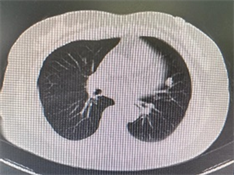
例1:女性,54岁,2月余前因高血压于外院就诊,查胸部CT示右肺下叶背段磨玻璃样小结节(图1(a)),大小约0.9 cm × 0.5 cm。无咳嗽、咳痰,无胸痛、胸闷等不适。既往有“高血压”、“冠心病”病史。于2023年3月25日胸腔镜下行右肺下叶楔形切除术。例2:女性,53岁,因胸痛行胸部CT,提示左肺上叶结节状高密度影(图1(b))。于2023年6月28日胸腔镜下行左肺上叶切除术 + 胸膜黏连烙断术 + 纵膈肺门淋巴结清扫术。
 (a) (b)
(a) (b)
Figure 1. CT (a) (Case 1) shows ground glass like small nodule in the dorsal segment of the lower lobe of the right lung; (b) (Case 2) shows nodular high-density shadows in the left upper lobe of the lung
图1. CT (a) (例1)示右肺下叶背段磨玻璃样小结节;(b) (例2)示左肺上叶结节状高密度影
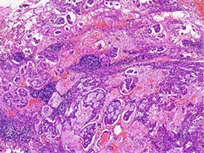
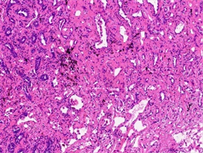
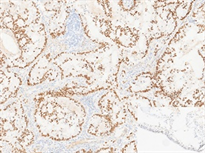
病理检查:眼观:例1右肺下叶组织一块,体积4 cm × 1.5 cm × 2 cm,切面见一结节,直径约0.5 cm,位于胸膜下。例2左肺上叶组织一块,体积15 cm × 11 cm × 2.5 cm,切面见一灰白灰褐色结节,体积1.5 cm × 1 cm × 1.2 cm。另送淋巴结数枚。镜检:例1低倍镜下肿瘤组织排列呈腺管状排列,腺腔内可见黏液分泌,局灶见淋巴细胞浸润,高倍镜下主要由立方细胞、纤毛细胞及基底细胞构成,部分区域双层结构不明显(图2(a))。例2低倍镜下肿瘤组织呈腺样、乳头状、筛状排列,边缘可见跳跃式生长,腺腔内富含黏液,背景可见淋巴细胞灶状浸润(图2(b)),高倍镜下主要由纤毛细胞、黏液细胞构成,未见明显基底细胞,可见核内包涵体(图2(c))。淋巴结25枚均呈反应性增生。免疫表型:CK7、TTF-1 (图3(a))、NapsinA阳性,基底细胞p40、p63、CK5/6阳性(例1 p63基底细胞无缺失,图3(b);例2仅p63强弱不等,图3(c)),p53呈野生型表达,EGFR阳性,Ki-67阳性指数分别约1%和10%,例2ALK (D5F3)阳性、PD-L1 (SP263) TPS值 < 1%。

 (a) (b) (c)
(a) (b) (c)
Figure 2. HE (a) (Case 1) shows mucus visible in the glandular lumen, with some areas having indistinct double-layer structures (100×); (b) (Case 2) shows edge jumping growth, with sieve like, papillary, and glandular structures. The glandular cavity is rich in mucus, and lymphocytes infiltrate in a focal pattern (40×); (c) (Case 2) shows no obvious basal cells were observed, but cilia (black arrow) and intranuclear inclusion bodies (white arrow) were visible (400×)
图2. HE (a) (例1)腺体管腔内可见黏液,部分区域双层结构不明显(100×);(b) (例2)边缘跳跃式生长,筛状、乳头状、腺样结构,腺腔内富含黏液,淋巴细胞灶状浸润(40×);(c) (例2)未见明显基底细胞,可见纤毛(黑色箭头)及核内包涵体(白色箭头) (400×)

 (a) (b) (c)
(a) (b) (c)
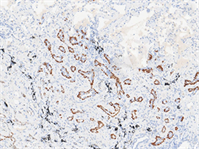
Figure 3. IHC. (a) (Case 1) p63 shows the complete double-layer structure (EnVision method, 100×); (b) (Case 2) TTF1 tumor tissue showed positive staining of ciliated cells, while the background normal bronchioles in the lower right corner showed positive staining of basal cells (EnVision method, 100×); (c) (Case 2) Unequal expression of p63 in basal cells (EnVision method, 100×)
图3. 免疫组化。(a) (例1) p63示完整双层结构(EnVision法,100×);(b) (例2) TTF1肿瘤组织纤毛细胞染色阳性,右下角背景正常细支气管显示基底细胞阳性(EnVision法,100×);(c) (例2)基底细胞p63强弱不等表达(EnVision法,100×)
病理诊断:1) (右肺下叶)细支气管腺瘤;2) (左肺上叶)非典型细支气管腺瘤。
随访结果:患者术后恢复良好,随访至2023年9月4日未复发。
2. 讨论
纤毛黏液结节性乳头状肿瘤(ciliated muconodular papillary tumor, CMPT)是一种肺部罕见的倾向良性的肿瘤,2002年首次由Ishikawa [1] 报道。随着CT影像检查的普及,越来越多的病例被报道,人们对它的认识也不断深入。2018年Chang等 [2] 发现了CMPT的形态特征及免疫表型与细支气管相近,扩充了CMPT的概念,引入了细支气管腺瘤(bronchiolar adenoma, BA)这一诊断术语。WHO (2021)胸部肿瘤分类中收录了这一类肿瘤,并命名为细支气管腺瘤/纤毛黏液结节性乳头状肿瘤 [3] 。
细支气管腺瘤被定义为一种来源于细支气管黏膜上皮的肺部肿瘤,由含有连续基底细胞层的温和的双层细支气管型上皮呈结节状增生形成。近端型BA模仿具有丰富黏液和纤毛细胞的近端细支气管,远端型模仿呼吸性细支气管的结构,缺乏黏液和纤毛细胞,甚至仅为II型肺泡上皮或Clara细胞。近端型BA腔面细胞TTF-1阴性或少数弱阳性,远端型TTF-1阳性,基底细胞表达p40 (或p63)、CK5/6。
但在部分细支气管腺瘤病例中,我们常会遇到基底细胞部分消失甚至完全消失的病例(特别是远端型BA),其腔面细胞符合经典型BA形态学特征,但部分区域缺乏基底细胞,也不能用“恶性”解释,称之为非典型BA [4] [5] 。Shao [6] 等通过对20例BA进行基因测序发现EGFR第20号外显子插入存在于100%的基底细胞完全缺失的病例、37.5%的非典型BA和8%的经典型BA中。因此认为BA从基底细胞无缺失(经典型BA),到基底细胞部分缺失(非典型BA),到完全缺失,可能是一组谱系性病变,基底细胞逐步缺失提示恶性转化。近年来陆续有BA恶变的病例报道 [5] [7] [8] [9] [10] 。
本文中例1的组织学表现以腺样结构为主,结合免疫组化染色可以确定属于远端型。例2的组织学特征与近端细支气管类似,但基底细胞仅p63强弱不等表达,并见部分腺体结构复杂,呈筛状改变,边缘呈跳跃式生长,可见纤毛及核内包涵体。TTF-1染色显示肿瘤组织纤毛细胞染色阳性,背景正常细支气管显示基底细胞阳性。分化成熟的纤毛细胞一般不会表达TTF-1,而掺杂在其他细胞中尚未分化成熟的纤毛细胞有时可以表现为TTF1弱阳性 [11] 。BA中Ki-67阳性指数一般 < 5%,PD-L1不表达;伴发腺癌时PD-L1、B7H3和B7H4表达水平升高 [7] [8] 。Ki-67、PD-L1高表达可能与BA恶性转化有关。目前例2的生物学行为不明,恶性潜能未定。
细支气管腺瘤容易与粘液腺癌相混淆,尤其在术中冰冻病理诊断时,仔细观察发现肿瘤组织临近细支气管动脉束或被中型动脉穿过,提示肿瘤与细支气管关系密切,需考虑BA的可能。BA需与以下肿瘤相鉴别:1) 浸润性黏液腺癌:主要与粘液丰富的近端型BA相鉴别。两者均沿肺泡壁生长,细胞异型性较小,可呈跳跃式生长,最为主要的鉴别点是BA存在基底细胞层和纤毛细胞。由于冷冻切片的原因,纤毛可能表现为游离缘的皱褶或顶端胞质突 [11] ,BRAF V600E是显示纤毛的较为可靠的标记 [12] 。2) 微浸润腺癌:远端型BA可出现基底细胞的缺失,由单层上皮构成,有时间质增宽,与腺癌的鉴别点在于前者的单层结构与具有双层结构的区域相移行,细胞异型性轻微,后者仅由异型肺泡上皮构成。3) 细支气管周围化生(peribronchiolar metaplasia, PBM):是一种相对常见的反应性病变,与吸烟 [13] 关系密切,两者组织学相似,不同之处在于BA主要是单发的结节样病灶,而PBM的病变通常为界限不清的小而多的点状病灶,几乎不形成独立性结节。4) 乳头状腺癌:近端型BA需与伴纤毛形成的乳头状腺癌鉴别,但后者仅由肿瘤性纤毛柱状上皮构成,可见核分裂像,缺乏基底细胞,具有恶性生物学行为 [14] 。5) 肺黏液表皮样癌(pulmonary mucoepidermoid carcinoma, PMEC):易与低级别PMEC相混淆,两者均含有粘液细胞,可以分泌大量黏液。但BA没有表皮样细胞和中间型细胞,也不具有PMEC特征性的MAML2基因重排。
细支气管腺瘤较为少见,并且容易与粘液腺癌相混淆,尤其在术中冰冻时。通过本文报道的2例BA的复习,了解其病理形态及免疫表型特征,避免误诊。
基金项目
1) 陕西省重点研发项目(No. 2023-YBSF-668)。
2) 陕西省人民医院领军人才支持计划项目(No. 2021LJ-12)。
NOTES
*通讯作者。