摘要: 目的:探讨蟛蜞菊内酯(Wedelolactone)在重症急性胰腺炎(SAP)大鼠肠屏障损伤中的作用及机制。方法:选取SPF级健康雄性Wistar大鼠40只,随机分为4组:假手术组(SO组)、重症急性胰腺炎组(SAP组)、蟛蜞菊内酯25 mg/kg治疗组(Wed-L)和蟛蜞菊内酯50 mg/kg治疗组(Wed-H)。采用逆行胆胰管穿刺法,向胆胰管内注入5%牛黄胆酸钠溶液(1 ml/kg)诱导SAP模型。假手术组给予等量的生理盐水。Wed-L组和Wed-H组在SAP模型建立后在1小时和6小时后给予腹腔注射25和50mg/kg的蟛蜞菊内酯。制模24 h后,再次麻醉大鼠。使用自动生化分析仪检测血液中的血清淀粉酶和脂肪酶水平及血清肿瘤坏死因子-α、白细胞介素-6,白细胞介素-18和白细胞介素-1β均使用标准诊断试剂盒进行检测;HE用于评估胰腺、肠道病理变化,并进行病理评分;通过用Western blot技术测定肠组织中细胞焦亡相关蛋白GSDMD、Caspase-11和紧密连接蛋白ZO-1、Occludin、Claudins-1的表达;RT-PCR检测细胞焦亡相关基因GSDMD和Caspase-11的表达情况。使用免疫荧光染色法测定肠上皮细胞细胞焦亡相关蛋白GSDMD、Caspase-11和紧密连接蛋白ZO-1、Occludin、Claudins-1的分布情况。结果:HE染色结果示,与SO组相比,SAP组出现明显肠道病理损伤(P < 0.01),而Wed-H组肠道损伤明显低于SAP组(P < 0.01);同样,SAP组胰腺损伤明显高于SO组(P < 0.01),Wed-H组的损伤较于SAP组的胰腺损伤减轻(P < 0.05),Wed-L和Wed-H之间的差异无统计学意义。SAP组的血清淀粉酶和脂肪酶水平明显高于SO组(P < 0.01),Wed-L和Wed-H组明显低于SAP组(P < 0.01)。SAP组TNF-α、IL-6、IL-1β、IL-18水平明显高于SO组(P < 0.01),Wed-L和Wed-H组明显低于SAP组(P < 0.01)。RT-PCR结果显示SAP组肠道组织GSDMD和Caspase-11的表达水平较SO组明显升高(P < 0.01),Wed-H组的表达水平明显低于SAP组(P < 0.01)。Western blot显示,SAP组肠道组织GSDMD和Caspase-11蛋白表达水平较SO组升高(P < 0.05)。与SAP组相比较,Wed-H组肠道组织GSDMD和Caspase-11蛋白表达水平明显下降(P < 0.05)。SAP组肠道组织ZO-1、Claudin-1、Occludin蛋白表达水平较SO组降低(P < 0.01)。与SAP组相比较,Wed-H组肠道组织ZO-1、Claudin-1、Occludin蛋白表达水平明显升高(P < 0.01)。免疫荧光结果显示SAP组GSDMD明显高于SO组和Wed-H组,而紧密连接蛋白ZO-1、Occludin、Claudin-1显示较SO组弱,Wed-H组较SAP明显改善。结论:蟛蜞菊内酯通过抑制Caspase-11下调GSDMD表达,减少其介导的细胞焦亡炎性因子的释放,减轻肠粘膜损伤。
Abstract:
Objective: To explore the role and mechanism of Wedelolactone in intestinal barrier injury in rats with severe acute pancreatitis (SAP). Methods: Forty SPF healthy male Wistar rats were randomly divided into four groups: sham operation group (SO group), severe acute pancreatitis group (SAP group), wedelolactone 25 mg/kg treatment group (Wed-L) and wedelolactone 50 mg/kg treatment group (Wed-H). SAP model was induced by retrograde cholangiopancreatography by injecting 5% sodium taurocholate solution (1 ml/kg) into cholangiopancreatography. The sham operation group was given the same amount of normal saline. Wed-L group and Wed-H group were given intraperitoneal injection of 25 and 50 mg/kg wedelolactone 1 hour and 6 hours after the estab-lishment of SAP model. After 24 hours of modeling, the rats were anesthetized again. The levels of serum amylase and lipase in blood and serum tumor necrosis factor-α, interleukin-6, interleukin-18 and interleukin-1β were detected by automatic biochemical analyzer, and standard diagnostic kits were used. HE was used to evaluate the pathological changes of pancreas and intestine, and made pathological score. The expressions of apoptosis-related proteins GSDMD, Caspase-11 and tight junction proteins ZO-1, Occludin and Claudins-1 in intestinal tissues were determined by Western blot. RT-PCR was used to detect the expression of apoptosis-related genes GSDMD and Caspase-11. The distribution of apoptosis-related proteins GSDMD, Caspase-11 and tight junction proteins ZO-1, Occludin and Claudins-1 in intestinal epithelial cells was determined by immunofluorescence staining. Results: HE staining showed that compared with SO group, SAP group had obvious intestinal pathological injury (P < 0.01), while Wed-H group had significantly lower intestinal injury than SAP group (P < 0.01). Similarly, the pancreatic injury in SAP group was significantly higher than that in SO group (P < 0.01), and the pancreatic injury in Wed-H group was less than that in SAP group (P < 0.05). There was no significant difference between Wed-L and WED-H. Serum amylase and lipase levels in SAP group were significantly higher than those in SO group (P < 0.01), while those in Wed-L and Wed-H groups were significantly lower than those in SAP group (P < 0.01). The levels of TNF-α, IL-6, IL-1β and IL-18 in SAP group were significantly higher than those in SO group (P < 0.01), while those in Wed-L and Wed-H groups were significantly lower than those in SAP group (P < 0.01). RT-PCR results showed that the expression levels of GSDMD and Caspase-11 in intestinal tissues in SAP group were significantly higher than those in SO group (P < 0.01), and the expression levels in Wed-H group were significantly lower than those in SAP group (P < 0.01). Western blot showed that the expression levels of GSDMD and Caspase-11 protein in intestinal tissues of SAP group were higher than those of SO group (P < 0.05). Compared with SAP group, the expression levels of GSDMD and Caspase-11 protein in intestinal tissue of Wed-H group decreased significantly (P < 0.05). The expression levels of ZO-1, Claudin-1 and Occludin in intestinal tissues in SAP group were lower than those in SO group (P < 0.01). Compared with SAP group, the expression levels of ZO-1, Claudin-1 and Occludin in intestinal tissue in Wed-H group were significantly higher (P < 0.01). Immunofluorescence results showed that GSDMD in SAP group was significantly higher than that in SO group and Wed-H group, while tight junction proteins ZO-1, Occludin and Claudin-1 were weaker than that in SO group, and Wed-H group was significantly improved than that in SAP. Conclusion: Wedelolactone down-regulates the expression of GSDMD by inhibiting Caspase-11, reduces the release of inflammatory factors in pyroptosis mediated by Caspase-11, and reduces intestinal mucosal injury.
1. 引言
急性胰腺炎(acute pancreatitis, AP)是一种突发的胰腺炎症,是消化系统疾病住院的主要原因之一 [1] 。当患者出现持续性的器官衰竭,则发展为重症急性胰腺炎(severe acute pancreatitis, SAP),胰腺继发感染是最严重的相关并发症,是高死亡率的主要原因 [2] 。SAP时患者肠屏障功能受损,细菌移位进入全身循环,进而导致继发感染,发展至全身炎症反应,甚至多器官功能障碍 [3] 。蟛蜞菊内酯(wedelolactone, Wed)是一种植物来源的香豆素,可以直接抑制IKK复合物来抑制caspase-11表达,具有抗肿瘤、抗骨质疏松、抗纤维化、抗丙型肝炎病毒和降低胆固醇等多种活性 [4] 。细胞焦亡是一种由炎性半胱天冬酶诱导的坏死和炎性程序性细胞死亡。细胞焦亡通过caspase-1依赖性或caspase-1非依赖性机制来调节。Caspase-1非依赖性细胞焦亡由人caspase-4、caspase-5或小鼠caspase-11执行,表现为细胞膜破裂,导致细胞的细胞质内容物释放,包括促炎细胞因子、内源性配体和其他危险相关分子模式 [5] 。有研究表明,感染性炎症患者肠道中广泛存在炎症性半胱天冬酶caspase-11激活 [6] 。已有证明,细胞焦亡与许多炎症性疾病有关,如败血症,哮喘,脑出血等 [7] ,Liu等人通过实验证明抑制细胞焦亡改善了败血症诱发的心肌病 [8] ;Pan等人通过实验证明抑制细胞焦亡可以减轻脑出血后的神经炎症反应 [9] ;Liu等人通过研究发现抑制细胞焦亡减轻哮喘患者的中性粒细胞气道炎症 [10] 。然而非经典途径细胞焦亡在SAP肠屏障损伤中的作用尚未可知,因此,我们推测抑制caspase-11介导的细胞焦亡可以为SAP的治疗提供新的方向。本实验通过逆行穿刺胆管,向胆胰管内注入5%牛磺胆酸钠构建大鼠SAP模型,检测肠道GSDMD和紧密连接蛋白的表达,并通过蟛蜞菊内酯抑制caspase-11降低GSDMD的表达,从而探讨非经典途径细胞焦亡对SAP大鼠肠道功能的影响。
2. 材料与方法
2.1. 实验动物与分组
8周龄SPF级雄性Wistar大鼠40只,体重250~300 g,由北京斯贝福生物技术有限公司提供,青岛大学中心实验室饲养。将大鼠圈养在12小时光照/黑暗循环下,22℃ ± 1℃、和45%~55%的湿度。动物护理和处理程序遵循实验动物护理和使用指南,并经青岛大学动物福利伦理委员会批准。按照随机数字表法将大鼠分为假手术组(SO),SAP模型组,SAP Wed-H组,SAP Wed-L组,每组10只。
2.2. 主要实验试剂
牛黄胆酸钠购自美国Sigma公司,蟛蜞菊内酯购自美国MedChemexpress公司,RNA提取试剂购自美国Thermo Fisher公司,反转录和荧光定量试剂盒购自日本TaKaRa公司,GSDMD抗体、Caspase-11抗体购自美国Proteintech Group公司,ZO-1抗体、Occludin抗体和Claudin-1抗体购自美国Abcam公司,血清肿瘤坏死因子-α (TNF-α)、白细胞介素(interleukins,IL-6、IL-1β和IL-18)酶联免疫吸附试验(enzyme linked immunosorbent assay, ELISA)检测试剂盒购自武汉Boster公司。
2.3. 动物模型制备
实验动物均于实验前禁食12 h,自由饮水。大鼠称重麻醉后夹住肝门部胰胆管,将连接微量泵的24 G套管针逆行穿进胰胆管,通过标准压力控制向胰胆管内注入5%牛磺胆酸钠(1 ml/kg)诱导SAP模型。假手术组的大鼠接受剖腹手术和相同体积的盐水溶液。Wedelolactone (SAP + Wed)治疗组的大鼠在手术后1小时和6小时通过腹膜内注射接受25 mg/kg或50 mg/kg wedelolactone。
2.4. 检测指标及方法
各组于制膜后24小时麻醉后取下腔静脉血;采取颈椎脱臼法处死大鼠,收集胰腺、回肠组织于4%多聚甲醛溶液中固定,用于组织学分析,其余部分于−80℃保存备用。
1) 血清脂肪酶和淀粉酶:使用自动分析仪测定血液样本中的血清淀粉酶(amylase, AMY)和脂肪酶(lipase, LIPA)的活性。
2) 胰腺病理学观察及病理评分:取胰腺组织,置于4%甲醛溶液中固定,常规进行脱水、石蜡包埋、切片,苏木素-伊红(hematoxylin-eosin, HE)染色,中性树脂封片,光镜下观察。采用双盲法,按Schmidt等提出的胰腺组织病理评分标准评分 [11] 。
3) 肠道病理学观察及病理评分:取回肠组织,同上法HE染色,200倍光镜下观察组织切片。按照Chiu分级评分标准进行小肠组织病理评分 [12] 。
4) 酶联免疫吸附测定(ELISA)分析:严格按照试剂盒说明书步骤操作,通过相应试剂盒检测血清和细胞上清液中TNF-α,IL-6,IL-1β,IL-18的水平。
5) 蛋白质免疫印迹实验(Western blott)检测肠组织GSDMD、caspase-11与TJ蛋白:通过RIPA裂解缓冲液从回肠组织中分离蛋白质,然后通过BCA蛋白质测定试剂盒测定蛋白质浓度。将蛋白质样品加入制备好的凝胶孔中进行电泳分离,然后转移到聚偏氟乙烯膜上。用5%脱脂牛奶封闭细胞膜1 h,并在4℃与一抗孵育过夜,最后在室温下与相应二抗孵育1 h,进行条带显影。应用Image-J软件对Western blot条带的密度进行定量。
6) 反转录–聚合酶链反应(RT-PCR)检测肠道GSDMD和caspase-11的表达:通过使用TRIzol试剂提取总RNA,将提取的RNA用紫外分光光度计进行检测,测量其在260 nm/280 nm波长处OD值,判断纯度。按照TaKaRa试剂盒要求进行反转录合成cDNA,以β-肌动蛋白(β-actin)为内参,,按照2-ΔΔCt法计算GSDMD和caspase-11的表达量。
7) 免疫荧光法测定TJ蛋白和GSDMD表达:取回肠组织,石蜡包埋、切片,烘干、水化,抗原热修复,用山羊血清封闭,加入一抗4℃孵育过夜,二抗孵育,DAPI核染色,滴加抗荧光淬灭剂封片,荧光显微镜下观察并拍照。
2.5. 统计学方法
数据表示为平均值 ± SD。使用GraphPad Prism软件版本8.0 (GraphPad, San Diego, CA, USA),使用单因素方差分析加Tukey多重比较检验或Kruskal-Wallis非参数检验分析组间差异。P < 0.05定义为差异具有统计学意义。
3. 结果
3.1. 胰腺和肠道病理学改变
光镜下显示(见图1),SO组大鼠胰腺(A)和肠黏膜(E)均未见明显损伤。SAP组(B)和Wed-H组(D)大鼠胰腺均有不同程度的水肿、出血及炎性细胞浸润,但Wed-H组病理学改变较SAP模型组明显减轻。SAP组(F)和Wed-H组(H)大鼠回肠组织也可见不同程度的损伤,Wed-H组损伤程度较SAP组有所减轻。SAP组大鼠肠黏膜内可见绒毛破损,部分可见局部出血,炎性细胞浸润,间质水肿;Wed-H组大鼠肠壁绒毛上皮下间隙增大,伴血管淤血,炎性细胞浸润,肠壁水肿。
定量分析显示(见图2):SAP组和Wed-H组大鼠胰腺组织与肠黏膜病理评分均明显高于SO组(P < 0.01),但Wed-H组病理评分明显低于SAP组(P < 0.01)。Wed-L和SAP组之间损伤无统计学意义(P > 0.05),Wed-H和Wed-L之间损伤无明显差异(P > 0.05)。
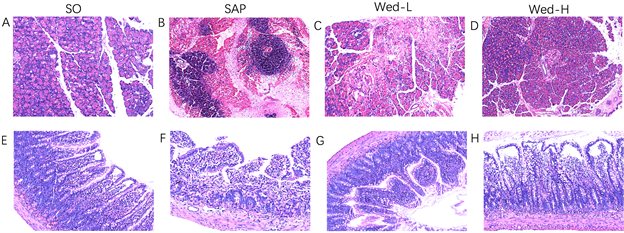
Figure 1. Pathological changes of pancreas and small intestine of rats in each group (×400 times). Normal pancreatic cells and normal intestinal epithelial cells can be seen in SO group. In SAP group, diffuse edema, interlobular space widening, inflammatory cell infiltration and partial parenchymal hemorrhage were observed. It is obvious that intestinal villi rupture and inflammatory cell infiltration in the intestine. The number of pancreatitis cells in Wed-H group was lower than that in SAP group. The epithelial-subcutaneous space of intestinal villi was enlarged, with partial edema and inflammatory cell infiltration, but the degree was less than that of SAP model group
图1. 各组大鼠胰腺、小肠HE染色病理情况(×400倍)。SO组可见正常胰腺细胞和正常肠道上皮细胞。SAP组可见弥漫性水肿,小叶间隙增宽,炎性细胞浸润,部分实质出血;肠道绒毛断裂,炎性细胞浸润。Wed-H组胰腺炎性细胞数量较SAP组减少;肠道肠壁绒毛上皮下间隙增大,部分水肿,炎性细胞浸润,但程度较SAP模型组减轻
3.2. 肠道组织GSDMD及Caspase-11表达(见图3)
通过PCR检测细胞焦亡非经典途径GSDMD和Caspase-11的表达,结果分析显示与SO组相比,SAP回肠组织中细胞焦亡相关基因GSDMD和Caspase-11的mRNA水平明显升高(P < 0.05);相比于SAP组,Wed-H组上述指标明显降低(P < 0.05)。Wed-L组和SAP组之间差异无统计学意义。
3.3. 肠道组织TJ蛋白和细胞焦亡蛋白表达(见图4)
用免疫印迹法检测各组大鼠回肠GSDMD、Caspase-11和TJ蛋白的表达。与SO组相比,SAP组的ZO-1、occludin和claudin-1的表达显著降低(P < 0.01),GSDMD、Caspase-11的表达显著升高(P < 0.01);与SAP组相比,Wed-H组的ZO-1、occludin和claudin-1的表达显著升高(P < 0.01),GSDMD、Caspase-11的表达显著降低(P < 0.01)。

Figure 2. Quantitative analysis of pathological scores of pancreatic and intestinal tissues. Note: **, P < 0.01 compared with SO group; ##, compared with SAP group, P < 0.01; #, compared with SAP group, P < 0.05
图2. 胰腺、肠道组织病理评分定量分析。注:**,与SO组相比P < 0.01;##,与SAP组相比P < 0.01;#,与SAP组相比P < 0.05
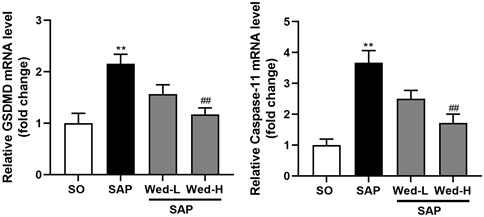
Figure 3. Relative quantitative analysis of GSDMD and Caspase-11. Note: **, P < 0.01 compared with SO group; ##, P < 0.01 compared with SAP group
图3. GSDMD、Caspase-11相对定量分析。注:**,与SO组相比P < 0.01;##,与SAP组相比P < 0.01
3.4. 细胞因子水平(见图5)
全自动生化仪检测脂肪酶和淀粉酶示SAP组、Wed-H和Wed-L组血清脂肪酶和淀粉酶水平明显高于SO组。酶联免疫吸附法检测血清中TNF-α、IL-6、IL-18 和IL-1β浓度,并对其进行定量分析,结果显示为在SAP组大鼠中TNF-α、IL-6、IL-1β、IL-18 浓度较SO组上升(P < 0.05),Wed-H和Wed-L组TNF-α、IL-6、IL-1β、IL-18浓度较SAP组下降(P < 0.05)。
3.5. 肠道组织TJ蛋白和细胞焦亡蛋白的分布(见图6)
通过免疫荧光检测GSDMD和TJ蛋白在肠道中的表达,结果显示肠上皮各细胞之间检测到GSDMD (红色)、Claudin-1 (绿色)、ZO-1 (绿色)和Occludin (绿色)。SAP组有大量GSDMD (红色)聚集,表明细胞焦亡被激活。Wed-H组GSDMD浓度明显低于SAP组。SAP组Claudin-1、ZO-1和Occludin明显低于SO组。Wed-H组Claudin-1、ZO-1和Occludin较SAP组明显改善。
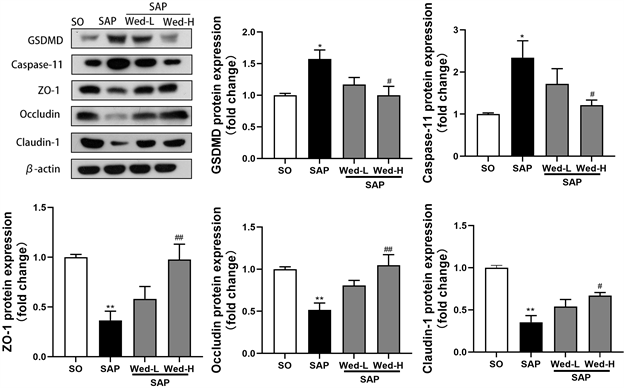
Figure 4. Results and relative quantitative analysis of intestinal TJ and cytokeratin. Note: **, P < 0.01 compared with SO group; ##, compared with SAP group, P < 0.01; #, compared with SAP group, P < 0.05
图4. 肠道TJ及细胞焦亡蛋白结果及相对定量分析。注:**,与SO组相比P < 0.01;##,与SAP组相比P < 0.01;#,与SAP组相比P < 0.05
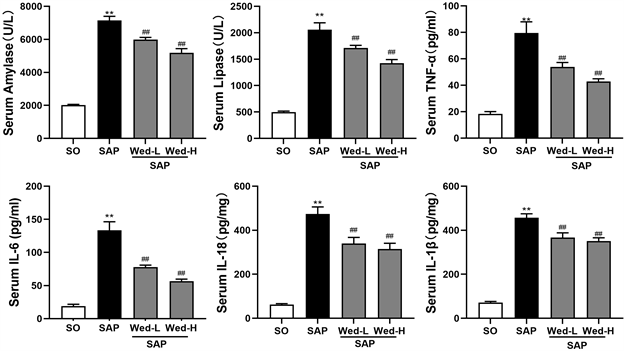
Figure 5. Relative quantitative analysis of cytokine levels. Note: **, P < 0.01 compared with SO group; ##, P < 0.01 compared with SAP group
图5. 细胞因子水平相对定量分析。注:**,与SO组相比P < 0.01;##,与SAP组相比P < 0.01
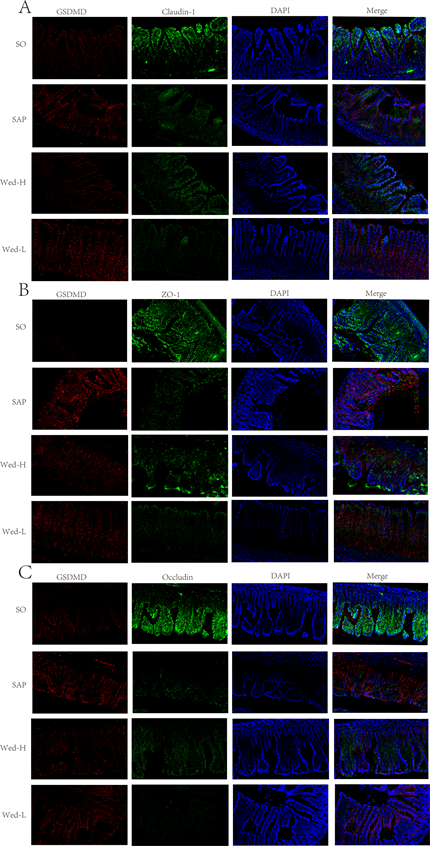
Figure 6. Distribution of GSDMD and TJ in intestinal epithelial cells of rats in each group (×200 times)
图6. 各组大鼠肠上皮细胞GSDMD和TJ的分布(×200倍)
4. 讨论
重症急性胰腺炎是一种高死亡率、发展迅速的疾病,其发病机制涉及一系列因素,尚未完全阐明 [13] 。SAP肠屏障功能受损,细菌移位进入全身循环,进而导致继发感染,发展至全身炎症反应,甚至多器官功能障碍,从而导致一系列并发症 [14] 。最近的研究表明,肠上皮细胞中存在着经典途径的细胞焦亡 [15] ,因此,我们通过SAP大鼠模型探讨非经典途径细胞焦亡即caspase-11在肠屏障功能中的作用。本研究观察了大鼠血清炎症指标水平及回肠组织形态学变化,表明caspase-11依赖性的细胞焦亡参与SAP的发展过程,其肠屏障功能明显受损。此外,本研究观察到通过蟛蜞菊内酯抑制caspases-11依赖性的细胞焦亡可以使以上损伤得到改善。
肠屏障是一种独特的粘膜屏障,既能消化和吸收营养,又能防御有害物质,如肠道有毒物质、病原体和抗原 [16] 。肠屏障由四部分组成:机械屏障、免疫屏障、化学屏障和生物屏障。完整的肠屏障在维持人体健康和治疗胃肠疾病甚至肠外疾病中起重要作用 [17] 。紧密连接,一种细胞连接,也是机械屏障的重要组成部分。TJ是由TJ蛋白复合物形成的高度动态结构,位于内皮细胞细胞间隙的外侧。TJ包括多种蛋白质,claudin和occludin是跨膜蛋白,ZO是细胞质中的衔接蛋白,它们的直接结合参与肠粘膜屏障完整性的调节 [18] 。本研究中通过免疫荧光检测ZO-1、Claudin-1和Occludin在肠道上皮的分布和通过免疫印迹检测肠道组织中TJ蛋白的含量来评估肠粘膜屏障功能,发现SAP大鼠ZO-1、Claudin-1和Occludin的表达下降,表明肠屏障功能受损。
细胞焦亡是一种程序性细胞死亡,然而其形态学不同于其他类型的程序性细胞死亡,如凋亡 [19] 。细胞凋亡有两张途径:外源性和内源性途径。每一种途径都需要特定的触发信号来启动能量依赖的分子事件级联,激活区自身的起始caspases (8, 9, 10),进而激活执行caspase-3。典型的凋亡细胞形态学包括细胞皱缩、染色质浓缩、脂质泡和凋亡小体 [20] 。与焦亡细胞相反,凋亡细胞保持着完整的细胞膜,细胞核受损而质膜保持完整 [21] 。细胞焦亡作为体内一种自然免疫反应,实际上更像是一把双刃剑。一方面,激活的炎症反应将病原体从宿主体内清除,并将感染的细胞转移到中性粒细胞和其他免疫细胞。由于炎症因子从其中释放,可以达到消灭感染性细菌和细胞内病原体的目的。另一方面,炎症反应的显著增加,不可避免地会导致致命损伤,如正常细胞死亡、组织损伤甚至器官衰竭等。当被脂多糖激活时,caspase-11可以诱导非经典途径细胞焦亡。活化的caspase-11催化GSDMD裂解成GSDMD-N并穿透细胞膜,导致细胞焦亡,促进IL-18和IL-1β的释放 [19] 。本实验中观察到SAP大鼠肠道组织中caspase-11和GSDMD蛋白显著增加,以及IL-18和IL-1β水平的显著升高。总之,以上观察结果表明,在SAP肠屏障损伤中发生了caspase-11介导的细胞焦亡。
有研究证明,Wed通过GPX4介导抑制细胞焦亡减轻急性胰腺炎相关肺损伤 [22] 。因此本实验中通过给予Wed,以确定抑制细胞焦亡能否改善SAP肠屏障损伤,结果发现SAP组GSDMD表达上升,而使用Wed可以降低GSDMD的表达并下降IL-1β和IL-18含量,提高了TJ蛋白的表达,结合观察到的免疫荧光结果,表明抑制细胞焦亡后,SAP大鼠炎症反应及程度明显降低,有效改善肠屏障损伤。
5. 结论
总之,在SAP肠屏障损伤大鼠中出现caspase-11依赖性细胞焦亡,通过Wed抑制caspase-11依赖性细胞焦亡可以减轻SAP肠屏障损伤并保护肠屏障功能。因此,靶向caspase-11依赖性细胞焦亡可能为治疗SAP肠损伤提供一种新的治疗靶点。
参考文献
NOTES
*通讯作者。