摘要: 目的:探讨miR-423-3p对乳腺癌细胞恶性生物学行为的影响及作用机制。方法:体外培养人乳腺癌细胞MDA-MB-231、MDA-MB-468、MCF-7、BT-549及正常乳腺上皮细胞MCF-10A,采用实时荧光定量PCR (RT-qPCR)法和Western Blot方法,分别检测miR-423-3p及CARNS1 mRNA和蛋白相对表达量。选取MCF-7细胞,将细胞分为转染mimics NC组(A组)、转染miR-423-3p mimics组(B组)、转染inhibitors NC组(C组)、转染inhibitors组(D组)。在239T细胞中分别转染并分为CARNS1-3'-UTR-wt+miR-423-3p mimics (E组)、CARNS1-3'-UTR-wt-mimics NC (F组)、CARNS1-3'-UTR-mut+miR-423-3p mimics (G组)及CARNS1-3'-UTR-mut+mimics NC (H组)。采用RT-qPCR和Western Blot法,检测A~D组细胞miR-423-3p及CARNS1 mRNA和蛋白表达情况,采用CCK8法、细胞划痕实验、Transwell法和流式细胞术检测细胞增殖、迁移、侵袭和凋亡情况。在线数据库预测miR-423-3p与CARNS1基因3'非翻译区(3'-UTR)的互补结合位点,双荧光素酶报告基因实验验证预测结果。结果:RT-qPCR结果显示,MCF-7、MDA-MB-231、MDA-MB-468及BT-549细胞中miR-423-3p的相对表达量均明显高于正常乳腺上皮细胞MCF-10A (P < 0.05),RT-qPCR和Western Blot检测结果显示,CARNS1 mRNA以及蛋白的相对表达量均明显低于正常乳腺上皮细胞MCF-10A (P < 0.05)。CCK8实验结果显示,在细胞培养的第24、48、72小时,B组细胞增殖活力明显高于A组,D组明显低于C组。细胞划痕实验显示,B组细胞愈合率明显高于A组(P < 0.05)。D组细胞愈合率明显低于C组(P < 0.05)。Transwell实验结果显示,过表达miR-423-3p后可促进乳腺癌细胞侵袭,而抑制miR-423-3p后会抑制乳腺癌细胞的侵袭,差异有统计学意义(P < 0.05)。流式细胞实验结果显示,B组较A组凋亡能力明显降低(P < 0.05),D组较C组凋亡能力明显增强(P < 0.05)。starBase数据库预测miR-423-3p与CARNS1基因3'非翻译区(3'-UTR)存在互补结合位点,双荧光素酶报告基因实验结果显示,E组较F组细胞相对荧光素酶显著下调(P < 0.05),G组与F组细胞间相对荧光素酶活性差异无统计学意义(P > 0.05)。RT-qPCR检测结果显示,B组细胞的miR-423-3p相对表达量明显高于A组(P < 0.05),而CARNS1 mRNA相对表达量明显低于A组(P < 0.05),D组细胞的miR-423-3p相对表达量明显低于C组(P < 0.05),而CARNS1 mRNA相对表达量明显高于C组(P < 0.05)。结论:miR-423-3p可通过靶向调控CARNS1促进乳腺癌细胞的恶性生物学行为。
Abstract:
Objective: To investigate the effect and mechanism of miR-423-3p on the malignant biological be-havior of breast cancer cells. Methods: Human breast cancer cells MDA-MB-231, MDA-MB-468, MCF-7, BT-549 and normal breast epithelial cells MCF-10A were cultured in vitro, and the relative mRNA and protein expressions of miR-423-3p and CARNS1 were detected by real-time quantitative PCR (RT-qPCR) and Western Blot, respectively. MCF-7 cells were divided into mimics NC transfection group (group A), miR-423-3p mimics group (group B), transfection inhibitors NC group (group C), and transfection inhibitors group (group D). 239T cells were transfected and divided into CARNS1-3'-UTR-wt miR-423-3p mimics (group E), CARNS1-3'-UTR-wt-mimics NC (group F), CARNS1-3'-UTR-mut miR-423-3p mimics (group G) and CARNS1-3'-UTR-mut mimics NC (group H). The mRNA and protein expressions of miR-423-3p and CARNS1 in group A~D cells were detected by RT-qPCR and Western Blot, and the proliferation, migration, invasion and apoptosis of cells were detected by CCK8 method, cell scratch assay, Transwell method and flow cytometry. The comple-mentary binding sites of miR-423-3p and the 3 untranslated region (3'-UTR) of CARNS1 gene were predicted by an online database, and the prediction results were verified by double luciferase re-porter gene assays. Results: RT-qPCR results showed that the relative expression levels of miR-423-3p in MCF-7, MDA-MB-231, MDA-MB-468 and BT-549 cells were significantly higher than those in normal mammary epithelial cells MCF-10A (P < 0.05), and the relative expression levels of mRNA and protein in CARNS1 were significantly lower than those in normal mammary epithelial cells (P < 0.05). The results of CCK8 experiments showed that at the 24th, 48th and 72nd hours of cell culture, the cell proliferation activity of group B was significantly higher than that of group A, and that of group D was significantly lower than that of group C. The cell scratch test showed that the cell healing rate of group B was significantly higher than that of group A (P < 0.05). The cell healing rate of group D was significantly lower than that of group C (P < 0.05). The results of Transwell assay showed that overexpression of miR-423-3p could promote the invasion of breast cancer cells, while inhibition of miR-423-3p would inhibit the invasion of breast cancer cells, and the difference was statistically significant (P < 0.05). The results of flow cytometry showed that the apoptosis ability of group B was significantly lower than that of group A (P < 0.05), and the apopto-sis ability of group D was significantly higher than that of group C (P < 0.05). The starBase database predicted that miR-423-3p had complementary binding sites in the 3' untranslated region (3'-UTR) of CARNS1 gene, and the results of double luciferase reporter gene assay showed that the relative luciferase of group E was significantly down-regulated compared with group F (P < 0.05), and there was no significant difference in relative luciferase activity between group G and group F (P > 0.05). The results of RT-qPCR showed that the relative expression of miR-423-3p in group B was signifi-cantly higher than that in group A (P < 0.05), while the relative expression of mRNA in CARNS1 was significantly lower than that in group A (P < 0.05), the relative expression of miR-423-3p in group D was significantly lower than that in group C (P < 0.05), and the relative expression of mRNA in CARNS1 was significantly higher than that in group C (P < 0.05). Conclusion: miR-423-3p can pro-mote the malignant biological behavior of breast cancer cells by targeting CARNS1.
1. 引言
乳腺癌(Breast Cancer, BC)是女性最常见的恶性肿瘤之一,其发病率和死亡率均位于女性癌症首位 [1] 。微小RNA (microRNA, miRNA)是一类长度为19~22个核苷酸的非编码RNA [2] ,可通过与靶基因mRNA的3'-UTR碱基互补配对,调控mRNA的翻译和降解,影响细胞的增殖、凋亡等生物学过程 [3] 。近年来相关研究表明,miRNA可以通过调控癌细胞中的各种代谢途径来调节基因表达,进而促进或抑制癌症进展 [4] 。miR-423-3p可通过靶向CYBRD1激活FAK信号通路促进肺腺癌上皮间质转化,进而促进肿瘤生长 [5] 。miR-423-3p可通过调控SUFU影响结肠癌细胞对5-Fu的敏感性 [6] ,此外,还有研究结果显示miR-423-3p在胶质瘤组织中显著上调,且miR-423-3p表达增加与胶质瘤患者的不良预后相关,并可通过下调PANX2促进胶质瘤的生长、进展 [7] 。然而,关于miR-423-3p在乳腺癌中的作用及机制研究尚未见报道,本文主要探究miR-423-3p对乳腺癌细胞增殖、迁移、侵袭和凋亡的影响,以及miR-423-3p与CARNS1基因的靶向调控关系,为乳腺癌的诊断和治疗提供新的靶点。
2. 材料和方法
2.1. 细胞与试剂
乳腺癌细胞MDA-MB-231、MDA-MB-468、MCF-7、BT-549及正常乳腺上皮细胞MCF-10A来自青岛大学医学部分子生物学与生物化学实验室。DMEM培养基、PBS、胰蛋白酶消化液、青/链霉素混合液购于上海源培生物科技有限公司,胎牛血清购于美国Gibco公司,CCK8试剂盒、多聚甲醛、结晶紫染液购于上海碧云天生物技术有限公司,逆转录试剂盒、Trizol购于南京诺唯赞生物科技公司,mimics及inhibitors转染试剂购于上海吉玛基因,CARNS1、GADPH抗体、二抗购于Proteintech公司,双荧光素酶报告基因检测盒购于北京全式金生物技术有限公司。
2.2. 细胞培养及分组
将乳腺癌细胞MDA-MB-231、MDA-MB-468、MCF-7、BT-549及正常乳腺上皮细胞MCF-10A置于含有10%胎牛血清和1%青/链霉素混合液的DMEM培养基中,在37℃、5% CO2的细胞培养箱中培养,于细胞融合度达80%~90%时传代,取对数生长期的细胞,用于后续实验。
在6孔板中培养MCF-7细胞并孵育过夜,当细胞融合度达60%~80%时使用转染试剂进行转染,分为mimics NC组(A组)、转染miR-423-3p mimics组(B组)、转染inhbitors NC组(C组)、转染inhibitors组(D组)。
2.3. RT-qPCR检测miR-423-3p和CARNS1的表达
在MDA-MB-231、MDA-MB-468、MCF-7、BT-549、MCF-10A细胞悬液中加入Trizol试剂提取RNA,逆转录为cDNA并扩增。上机条件:① 在95℃下预变性3 min;② 95℃,15 s;55℃,20 s;72℃,15 s,循环40次。miR-423-3p的内参为U6,CARNS1的内参为GAPDH。采用2−ΔΔCt计算miR-423-3p和CARNS1的相对表达量。各引物序列见表1。
2.4. Western Blot检测CARNS1的表达
收集上述细胞于EP管中,加入裂解液提取蛋白,使用BCA试剂盒测定蛋白浓度,通过SDS-PAGE分离蛋白质并转移至PVDF膜,使用5%脱脂牛奶封闭1 h,加入内参GAPDH一抗(1:1000)、目的基因CARNS1一抗(1:5000),在4℃冰箱孵育过夜。使用TBST漂洗3次,每次10 min。然后加入二抗室温孵育1 h,再次用TBST洗膜3次,每次10 min。使用ECL化学发光,成像系统显影、拍照并测量灰度值。CARNS1蛋白相对表达量 = CARNS1蛋白条带灰度值/内参GAPDH蛋白条带灰度值。
2.5. CCK8实验
将转染后的细胞悬液接种至96孔板中(10 μL/孔),分别加入10 μL的CCK8溶液,置于细胞培养箱内孵育1 h后,使用酶标仪测定各孔在波长450 nm处的吸光值(A)。
2.6. 细胞划痕实验
将转染后的各组细胞接种于6孔板,待细胞生长至铺满整孔时,用200 μL移液管枪头沿直尺垂直划痕,后加入无血清培养基,在0和48 h使用显微镜拍摄记录。
2.7. Transwell检测实验
取对数生长期的细胞,用PBS和无血清培养基先后洗涤,使用无血清培养基培养,调整浓度至2 × 105/mL。将Matrigel胶加入Transwell小室的上室,加入150 μL细胞悬液,在下室加入800 μL含10%血清的培养基,置于培养箱培养24 h。使用4%多聚甲醛溶液固定,结晶紫染色。在倒置显微镜下计算中间和四周5个视野的细胞数,取平均值。
2.8. 流式细胞术
取转染后的细胞使用PBS洗涤3次,置于六个孔板中,每孔含有加入5 µL FITC抗体和5 µL PI抗体,室温下避光染色30 min,再次PBS洗涤细胞,使用流式细胞仪和BD Cell Quest Pro软件分析细胞凋亡的比例。
2.9. 双荧光素酶报告基因测定
starBase数据预测miR-423-3p与CARNS1基因的结合位点。构建CARNS1野生型(wt)和突变型(mut)重组质粒,将239T细胞接种于6孔板中培养,分别转染CARNS1-3'-UTR-wt+miR-423-3p mimics (E组)、CARNS1-3'-UTR-wt-mimics NC (F组)、CARNS1-3'-UTR-mut+miR-423-3p mimics (G组)及CARNS1-3'-UTR-mut+mimics NC (H组),24 h后,使用双荧光素酶报告基因试剂盒于酶标仪中测定荧光素酶活性。
2.10. RT-qPCR检测转染后细胞中miR-423-3p和CARNS1 mRNA和蛋白的表达
在转染后细胞中分别加入Trizol试剂提取RNA,逆转录为cDNA并扩增。miR-423-3p以U6为内参照,CARNS1以GAPDH为内参照,使用RT-qPCR检测转染后各组细胞miR-423-3p和CARNS1的表达,上机条件同前。各引物序列见表1。收集转染后的MCF-7细胞,加入裂解液提取蛋白,使用BCA试剂盒测定蛋白浓度,使用Western Blot检测各组转染后细胞中CARNS1蛋白的表达量。
2.11. 统计学分析
使用SPSS 26.0软件进行分析,所有实验均独立重复3次,结果取平均值。正态分布的计量资料以均数 ± 标准差(
± s)表示,两组比较采用t检验,多组间比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
3. 结果
3.1. miR-423-3p和CARNS1 mRNA及蛋白在乳腺癌细胞的表达情况
RT-qPCR实验结果表明,miR-423-3p在乳腺癌细胞MDA-MB-231、MDA-MB-468、MCF-7、BT-549中的表达较正常乳腺上皮细胞MCF-10A显著上调,表达量分别为4.012 ± 0.438,1.652 ± 0.111,5.983 ± 0.431,2.808 ± 0.267,1.000 ± 0.056,差异有统计学意义(P < 0.05),在MCF-7中表达最高。而CARNS1在乳腺癌中显著下调,上述各细胞系中CARS1 mRNA的表达量分别为0.449 ± 0.053,0.601 ± 0.056,0.174 ± 0.010,0.330 ± 0.020,1.000 ± 0.062 (P < 0.05),在MCF-7中表达最低。Western Blot结果显示,上述各细胞系中CARNS1蛋白相对表达量分别为0.660 ± 0.026,0.809 ± 0.022,0.278 ± 0.011,0.508 ± 0.028,1.000 ± 1.019,差异有统计学意义(P < 0.05)。
3.2. miR-423-3p对乳腺癌细胞增殖能力的影响
CCK-8实验结果显示,miR-423-3p mimics组(B组)较mimics NC组(A组)增殖活力明显升高,转染inhibitors组(D组)较转染inhbitors NC组(C组)组增殖活力明显降低,差异有统计学意义(P < 0.05)。见表2。
3.3. miR-423-3p对乳腺癌细胞迁移能力的影响
细胞划痕实验结果表明,mimics NC组(A组)、转染miR-423-3p mimics组(B组)、转染inhbitors NC组(C组)、转染inhibitors组(D组)细胞愈合率分别为39.540 ± 5.897,72.910 ± 3.292,39.750 ± 2.780,20.260 ± 1.789,其中miR-423-3p mimics组(B组)较mimics NC组(A组)愈合率明显升(P < 0.05),转染inhibitors组(D组)较转染inhbitors NC组(C组)组愈合率明显降低(P < 0.05)。见图1。

Table 2. Comparison of MCF-7 cell proliferation activity
表2. MCF-7细胞增殖活力比较
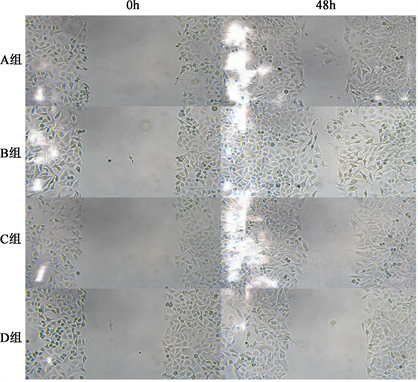
Figure 1. Comparison of the migration ability of MCF-7 cells in groups A~D after transfection
图1. 转染后A~D组MCF-7细胞迁移能力比较
3.4. miR-423-3p对乳腺癌细胞侵袭能力的影响
Transwell实验结果显示,过表达miR-423-3p后可促进乳腺癌细胞侵袭,而敲除miR-423-3p后会抑制乳腺癌细胞的侵袭,差异有统计学意义(P < 0.05)。见图2。
3.5. miR-423-3p对乳腺癌细胞凋亡的影响
流式细胞术结果显示,过表达miR-423-3p后可抑制乳腺癌细胞凋亡,而敲除miR-423-3p后会促进乳腺癌细胞的凋亡,差异有统计学意义(P < 0.05)。
3.6. miR-423-3p与CARNS1在乳腺癌中的靶向关系
通过starbase在线数据库预测miR-423-3p与CARNS1具有结合位点(图3)。双荧光素酶活性报告实验结果显示E~H组间细胞相对荧光素酶活性值差异有统计学意义(p < 0.05),其中E组较F组细胞相对荧光素酶显著下调(p < 0.05),G组与F组细胞间相对荧光素酶活性差异无统计学意义(p > 0.05)。见图4。
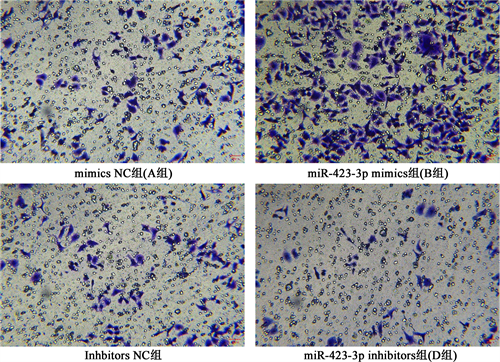
Figure 2. Comparison of the invasion ability of MCF-7 cells in groups A~D after transfection
图2. 转染后A~D组MCF-7细胞侵袭能力比较
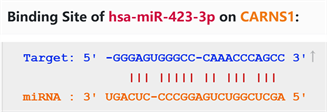
Figure 3. Predicted binding sites of miR-423-3p and CARNS1
图3. miR-423-3p与CARNS1的预测结合位点
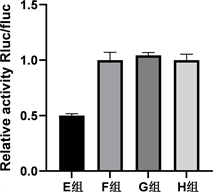
Figure 4. Dual luciferase activity reporter assay to detect the targeting relationship between miR-423-3p and CARNS1
图4. 双荧光素酶活性报告实验检测miR-423-3p与CARNS1的靶向关系
3.7. miR-423-3p抑制CARNS1在乳腺癌中的表达
RT-qPCR实验结果显示,A~D各组细胞miR-423-3p相对表达量分别为1.000 ± 0.014,3.168 ± 0.289,1.013 ± 0.064,0.457 ± 0.043,差异有统计学意义(P < 0.05)。其中,miR-423-3p mimics组(B组)较mimics NC组(A组)组显著升高,转染inhibitors组(D组)较转染inhbitors NC组(C组)组显著降低。上述各细胞系中CARS1 mRNA的表达量分别为1.000 ± 0.091,0.526 ± 0.019,1.008 ± 0.043,3.161 ± 0.142,差异有统计学意义(P < 0.05)。其中,miR-423-3p mimics组(B组)较mimics NC组(A组)组显著降低,转染inhibitors组(D组)较转染inhbitors NC组(C组)组显著升高。
4. 讨论
随着近年来医疗技术的飞速发展,包括手术、放疗、化疗、内分泌、靶向和免疫治疗等乳腺癌的诊治能力大幅提高,然而仍有部分患者病情进展迅速,预后较差 [8] 。因此,探索新的治疗方式对乳腺癌患者尤为重要。miRNA通过与mRNA的3'-UTR区结合发挥作用,促进其降解并抑制miRNA翻译,在细胞生长、分化、增殖、凋亡等阶段发挥重要作用 [9] [10] [11] [12] ,与乳腺癌的发生、发展密切相关 [13] 。本文的研究对象miR-423-3p在肺癌 [5] [14] 、肝癌 [15] 、结肠癌 [6] 、前列腺癌 [16] 、胶质瘤 [7] 等肿瘤进展可作为促癌基因,而在某些肿瘤中亦可作为抑癌基因 [17] 。本研究结果显示,miR-423-3p在乳腺癌细胞中高表达,通过CCK-8实验、细胞划痕实验、Transwell和流式细胞术等细胞功能实验证明,miR-423-3p促进乳腺癌细胞的恶性生物学行为,具有致癌基因的作用。
CARNS1 (肌肽合酶1)是一种蛋白质编码基因,主要参与肌肽生物合成和组氨酸降解 [18] 。肌肽最初是在骨骼肌中发现的,是一种天然存在的含有二肽的组氨酸,它可以作为细胞内pH缓冲剂和抗氧化剂 [19] 。熊峰等发现,CARNS1在冠心病中可能是一个保护性因素 [20] 。此外,还有研究显示注射肌肽可抑制大鼠脾脏的交感神经活动,减少大鼠结肠癌细胞的增殖 [21] 。本研究结果提示,CARNS1在乳腺癌细胞中表达降低。双荧光素酶基因报告实验验证了miR-423-3p与CARNS1的靶向关系,并通过RT-qPCR和Western Blot实验进一步验证,转染miR-423-3p mimics组miR-423-3p表达明显升高,而CARNS1 mRNA及蛋白表达水平显著降低;转染miR-423-3p inhibitors组miR-423-3p表达被显著抑制,CARNS1 mRNA及蛋白表达水平明显升高。由此可证明,CARNS1受到miR-423-3p的负向调控。
综上所述,本研究证明miR-423-3p是一个起促癌作用的miRNA,而CARNS1是一种抑癌基因。在乳腺癌细胞中,miR-423-3p通过抑制CARNS1的表达,促进乳腺癌细胞的增殖、迁移、侵袭并抑制其凋亡。由于miRNA参与调控多个靶基因及信号通路,miR-423-3p调控CARNS1促癌的具体机制及相关通路还需进一步研究。
NOTES
*通讯作者Email: wuliqd@163.com