1. 引言
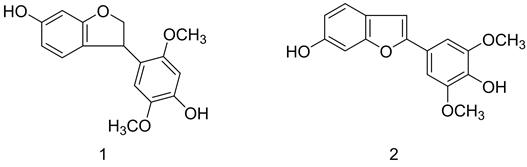
能量代谢失衡是肥胖和代谢综合征的主要原因,在调节细胞能量状态的蛋白激酶级联反应中,腺苷酸活化蛋白激酶(AMPK)是其中枢组成部分。AMPK信号通路是目前具有吸引力的治疗肥胖、胰岛素抵抗、2型糖尿病和其他代谢病的重要靶点[1] 。另一方面,苯并呋喃类化合物是一类具有很强生物活性的化合物,它广泛存在于天然和非天然产物中,其中2-芳基取代的苯并呋喃类化合物是苯并呋喃类天然产物中很重要的一大类,由于其多样的生物活性而备受关注。Erythribyssin H1是从大戟科大戟属植物玉鳞宝(Euphorbia globosa)的根的乙酸乙酯提取液中分离提取出的一个新的苯并呋喃类化合物,属于3-芳基取代2,3-二氢苯并呋喃类化合物。Nguyen等用免疫印迹分析法测定了在差异小鼠C2Cl2骨骼肌细胞的AMPK磷酸化水平,结果显示其AMPK激动活性较弱[2] 。
为了考察此类化合物的AMPK激动活性,本文设想:将Erythribyssin H 3位的芳基取代基移位至2位,同时将其的6’位甲氧基移位至5’位,最后得到了Erythribyssin H的类似物2-(4-羟基-3,5-二甲氧基苯基)-6-羟基苯并呋喃(2-(4-hydroxy-3,5-dimethoxyphenyl)-6-hydroxybenzofuran, 2),用以研究考察2的生物活性。
(2)的合成关键在于苯并呋喃环的构建和构成苯并呋喃环的两个关键中间体醛的合成,参考文献报道,使用合适的芳香族醛酮,经交叉的McMurry偶联反应得到邻乙烯基苯酚产物,然后通过氧化环合可以构建2-苯并呋喃环[3] 。根据2的化学结构,可以选择2,4-二取代苯甲醛和3,4,5-三取代苯甲醛作为交叉McMurry偶联反应的原料,逆合成分析见Scheme 1。
综上所述,本文首先分别以间苯二酚和丁香醛为起始原料,经Vilsmier-schmidet反应和酚羟基保护反应合成所需要的两个关键中间体醛2-羟基4-苄氧基苯甲醛4和3,5-二甲氧基4-苄氧基苯甲醛5。然后使用McMurry交叉偶联反应来进行两个关键中间体醛4和5的交叉偶联反应,得到邻乙烯基苯酚产物6,6在I2/K2CO3试剂作用下环合成为2-苯并呋喃环7,最后脱去苄基保护基得到目标化合物2。合成路线见Scheme 2。
2. 结果与讨论
由间苯二酚制备2,4-二羟基苯甲醛采用Vilsmier-Schmidet反应,我们采用了两种方法,并进行了
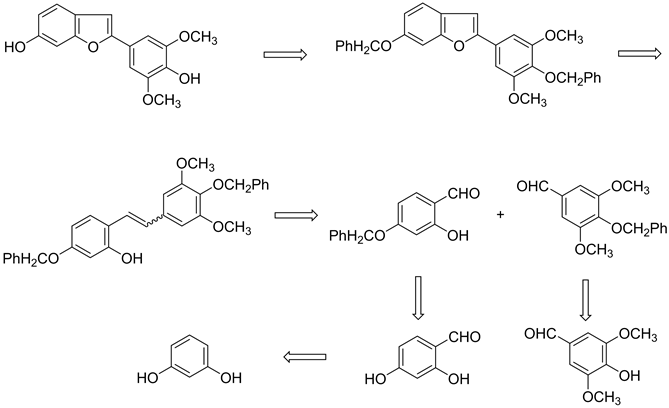
Scheme 1. Retro-synthetic analysis of compound 2
图1. 化合物2的逆合成分析

Scheme 2. Synthetic routes of the target compound 2
图2. 目标化合物2的合成路线
试验,即可以使用(COCl)2/DMF作为甲酰化试剂或者使用POCl3/DMF来制备Vilsmier试剂[4] [5] 。研究发现前者反应所形成的中间体8会从反应溶剂乙腈中析出,且反应液为很浓稠的固体,需要强力的搅拌来使转化完全,此法的收率只有60%;后者又称为Vilsmier-Haauc反应,反应过程中产生中间体9,水解得到3,此法收率88%。

由化合物3制备4虽然是属于酚羟基氧原子上的烷基化反应,但是由于3的分子上存在二个羟基而存在区域选择性的问题使得反应变得复杂化。而且,反应条件对3的区域选择性影响很大:或者4的收率很低,或者由于在苯环2-位同时被苄基化而使4的纯度很差[6] [7] 。文献报道以溴苄为原料,用三乙胺、吡啶等有机碱催化几乎不发生反应,而在DMF中用钠氢等强碱催化也只有50%的转化率[8] 。我们参照文献用氯苄为原料,碳酸氢钠为缚酸剂,加入少量的碘化钾催化,在乙腈中回流反应20小时,4的最终收率可达88.7%。
中间体6的合成采用McMurry交叉偶联反应,按照文献报道[9] ,使用Zn(10q)/TiCl4(5q)/THF反应体系,文献中所述金属催化剂的回流时间为2.5 h,而我们在实际实验操作时,TLC分析没有发现交叉偶联产物。故本文在保持加料比例不变的情况下,对金属催化剂(Zn/TiCl4)的回流时间做了一个简单考察,结果见表1。从表中可以看出,延长催化剂的回流时间,交叉偶联产物收率有比较大的提高。
关于化合物2的活性测试结果将另文报道。
3. 实验部分
熔点测试采用Tektronix X4熔点仪(温度计未经校正);1H-NMR用Bruker DRX-300核磁共振仪测定(溶剂CDCl3、DMSO,TMS(四甲基硅烷)内标);质谱由Esquie HCT PLUS色谱-质谱联用仪测定。
3.1. 2,4-二羟基苯甲醛(3)的制备
将间苯二酚5.5 g(0.05 mmol)溶于DMF 4.93 g(0.068 mmol)中,室温下将其加入150 ml的三颈瓶中,保持机械搅拌下,向反应体系中加入三氯氧磷5.35 ml(0.57 mmol),加完后继续搅拌反应2 h得到稠状液体。反应混合物用热的50% NaOAc溶液75 ml溶解,冷却后,混合液使用乙醚(3 × 30 ml)萃取,水洗,无水硫酸镁干燥。真空浓缩至干,残余物用水重结晶,干燥得到白色固体6.1 g,收率88.5%。m.p 134℃

Table 1. The reflux time investigation of cross McMurry coupling reaction
表1. McMurry交叉偶联反应催化剂回流时间考察
~136℃([5] :133℃~138℃)。1H-NMR (CDCl3, 300 MHz) δ 5.54(broad, H, OH),6.45(s, 1H, Ar-H),6.58(d, J = 7.2 Hz, 1H, Ar-H),7.55(d, J = 7.2 Hz, 1H, Ar-H),9.87(s, 1H),11.43(s, 1H)。
3.2. 2-羟基-4-苄氧基苯甲醛(4)的制备
将2,4-二羟基苯甲醛8 g(57.97 mmol)溶于200 ml乙醇中,室温下将其加入500 ml的三颈瓶中,再向反应体系中加入碳酸氢钠5.6 g(66.67 mmol)、碘化钾968 mg(5.8 mmol),搅拌5分钟后,冰浴条件下向反应体系中加入氯苄8.6 ml(75.36 mmol),之后,撤掉冰浴,加热回流反应20 h。冷至室温,真空浓缩反应液,向残余物中分别加入40 ml水,5 ml浓盐酸。用乙酸乙酯(3 × 100 ml)萃取混合液,收集有机层,无水硫酸钠干燥,真空浓缩,得到的残余物柱层析(SiO2,洗脱剂PE:EA = 4:1)得到白色固体11.72 g,收率88.7%。m.p 76℃~78℃([7] :74℃~76℃),m/z: 228 [M+H]+。
3.3. 4-苄氧基-3,5-二甲氧基苯甲醛(5)的制备
将丁香醛1 g(5.5 mmol),碳酸钾0.82 g( 5.94 mmol)和碘化钾0.25 g(1.51 mmol)悬溶于40 ml乙醇中,室温下将其加入100 ml单颈瓶中个,保持搅拌5分钟后,再向反应体系中加入氯化苄0.82 g(6.48 mmol),加热回流反应6 h,待反应原料消耗完全后,停止反应,冷至室温。向反应体系中加入3 ml水,真空蒸出溶剂乙醇。残余物倒入8 ml的1 M NaOH溶液中,析出固体,过滤,滤饼用冰水(3 × 5 ml)冲洗,收集滤饼,柱层析(SiO2,洗脱剂PE:EA = 5:1),得到黄色固体1.3 g,收率93%。1H-NMR (CDCl3, 300 MHz) δ 3.89(s, 6H, 2-CH3),5.13(s, 2H, -CH2-),7.11(s, 2H, Ar-H),7.33(m, 3H, Ar-H),7.46(m, 2H, Ar-H),9.86(s, 1H, -CHO)。m/z: 272 [M+H]+。
3.4. 3-苄氧基-6-(4’-苄氧基-3’,5’-二甲氧基苯乙烯基)苯酚(6)的制备
将活化锌粉2.89 g(44.12 mmol)悬溶于120 ml THF(无水无氧处理)中,室温下将其加入250 ml四颈瓶中,氮气保护。保持磁力搅拌下,用冰盐浴冷却反应体系至−5℃~0℃,并缓慢注射入四氯化钛2.4 ml(22.06 mmol),保持反应液温度<0℃。撤掉冰盐浴,将反应体系升至室温,搅拌反应0.5 h后,加热回流反应6 h后,反应体系冷至室温。再用冰盐浴使反应体系<0℃,加入2-羟基4-苄基苯甲醛1.0 g(4.41 mmol)和4-苄基2,3-二甲氧基苯甲醛1.0 g(3.68 mmol)的15 ml THF(无水无氧处理)溶液,加毕后,加热回流反应液1~2 h至原料完全消耗。反应体系冷至室温,加入8~10 ml 10%NaHCO3溶液,继续搅拌1 h,用硅藻土过滤混合液,CH2Cl2洗涤滤饼,收集滤液,无水硫酸钠干燥过夜,真空浓缩,得到的残余物柱层析(SiO2,洗脱剂PE:acetone = 15:1),得到微黄色结晶0.96 g,收率55.8%。1H-NMR (CDCl3, 300 MHz) δ 3.79(s, 3H, OCH3),3.81(s, 3H, OCH3),4.95(4H, 2-OCH2),6.50(m, 2H),6.66(d, J = 4.7 Hz, 2H),6.75(m, 1H),6.91(d, J = 4.7 Hz, 2H),7.26(m, 2H),7.30(m, 7H),7.34(m, 2H)。m/z = 468。
3.5. 2-(4’-苄氧基-3’,5’-二甲氧基苯基)-6-苄氧基苯并呋喃(7)的制备
将3-苄基-6-(4’-苄基-3’,5’-二甲氧基苯乙烯基)苯酚800 mg(1.71 mmol)溶于25 ml THF(无水处理)中,室温下将其加入100 ml单颈瓶中,连续搅拌下再向反应体系中加入碳酸钾1.31 g(9.49 mmol),搅拌反应10分钟后,加入I2 2.4 g(9.49 mmol),继续反应2~3 h至原料完全消耗。之后,反应液倒入8 ml饱和NaHCO3溶液中,再逐滴滴入饱和NaHSO3溶液将过量的I2消耗完,乙酸乙酯(3 × 20 ml)萃取混合液,收集有机层,无水硫酸钠干燥过夜,得到的残余物柱层析(SiO2,洗脱剂PE:acetone = 15:1),得到白色结晶672 mg,收率84%。1H-NMR (CDCl3, 300 MHz) δ 3.84(s, 3H, OCH3),3.90(s, 3H, OCH3),5.11(4H, 2-OCH2),6.65(m, 1H),6.78(d, 2H),7.04(d, 2H),7.39(m, 2H),7.52(m, 7H),7.63(m, 2H)。m/z = 496。
3.6. 2-(4’-羟基-3’,5’-二甲氧基苯基)-6-羟基苯并呋喃(2)的制备
将2-(4’-苄基-3’,5’-二甲氧基苯基)-6-苄基苯并呋喃93 mg(0.2 mmol)溶于20 ml CH2Cl2(无水处理)中,室温下将其加入到100 ml单颈瓶中,保持连续搅拌下,缓慢滴入四氯化钛0.05 ml(0.5 mmol),常温下搅拌反应2 h至原料完全消耗。向反应体系中加入10 ml甲醇终止反应,用乙酸乙酯(3 × 15 ml)萃取,收集有机相,无水硫酸钠干燥过夜,真空浓缩,得到的残余物柱层析(SiO2,洗脱剂PE:EA = 5:1)得到微黄色结晶51 mg,收率89.5%。1H-NMR (CDCl3, 300 MHz) δ 3.76(m, 6H, 2-OCH3),4.81(s, 1H, OH),5.60(s, 1H, OH),6.75(m, 2H),6.98(m, 3H),7.30(m, 1H)。m/z = 316,301,286。

NOTES
*通讯作者。