1. 引言
蛋白酪氨酸磷酸酶(PTPs)与蛋白酪氨酸激酶(PTKs)一起协同作用许多细胞活动 [1] [2]。目前,已有多种靶向PTKs的小分子抑制剂被FDA批准上市,而且还有许多小分子抑制剂正处于临床阶段。可惜的是尚未有靶向PTPs的抑制剂的药物上市。随着对PTPs家族酶及其抑制剂的研究,对PTPs调控的信号通路进一步了解,为相关疾病的治疗提供新的思路 [3]。
由PTPN11编码的SHP2,是一种在体内广泛存在的蛋白酪氨酸磷酸酶,调控细胞内的蛋白磷酸化水平,参与细胞生命活动 [4] [5]。位于细胞质中的SHP2可以被各种受体酪氨酸激酶募集来诱导细胞信号传导,并参与多个细胞内致癌信号传导级联反应,例如Jak/STAT [6] [7]、PI3K/AKT [8] [9]、RAS/Raf/MAPK [10] [11] [12]、PD-1/PD-L1 [13] [14] 和mTOR通路 [15]。SHP2的异常活化和突变与多种恶性肿瘤的发生、发展密切相关,此外对于血液瘤疾病的治疗药物尤其是对青少年粒单细胞白血病(JMML)和急性髓系白血病(AML)等罕见病缺乏有效的临床治疗手段。目前已被证实在白血病中的SHP2可作为肿瘤启动子 [16] [17],是一种潜在的富有前景的癌症治疗靶点 [18]。目前SHP2抑制剂取得重要进展 [19] [20],现在至少有三个抑制剂已在进行临床试验,包括Novartis公司的TNO155 [21],Revolution公司和Sanofi公司的RMC-463025 [22] 和Jacobio Pharmaceuticals公司的JAB-3068 [23]。对于许多与疾病密切相关突变型SHP2,仍然缺乏特异性抑制剂 [24] [25] [26] [27] [28]。因此,发现新型SHP2抑制剂,丰富SHP2抑制剂的多样性是十分有必要的。
在前期的工作中,我们发现了芳酰胺类SHP2抑制剂 [29]。如图1所示,为拓展SHP2抑制剂的多样性,我们设计并合成了18个化合物氨基苯甲酸衍生物,其结构经1HNMR分析测试确认,合成路线如图2所示。活性测试结果表明,部分化合物对SHP2在50 mM浓度下显示了一定强度的抑制活性。其中化合物15对在50 mM浓度下SHP2显示了48.51%抑制活性,可以作为后续SHP2抑制剂开发的活性片段分子。
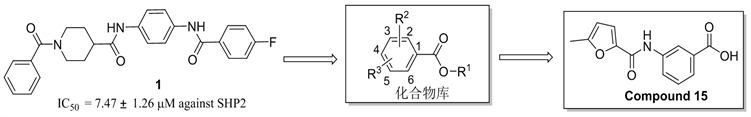
Figure 1. The design of target compounds
图1. 目标化合物的设计
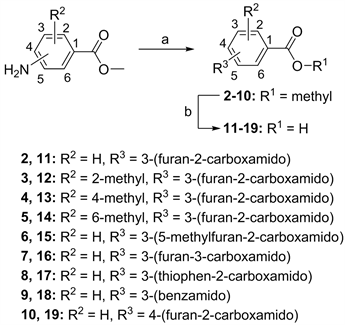 试剂和条件:(a) RCOOH, HATU, DIPEA, DMF, rt; (b) NaOH, MeOH, rt.
试剂和条件:(a) RCOOH, HATU, DIPEA, DMF, rt; (b) NaOH, MeOH, rt.
Figure 2. Synthetic route for compounds 2~19
图2. 化合物2~19的合成路线
2. 材料与方法
2.1. 材料
所有化学品均为购买且未有特殊说明均为化学纯。在Varian MR-400光谱仪上记录1H-NMR(400 MHz)光谱,使用DMSO(1H-NMR)的2.50信号作为内标。SHP2催化域蛋白由国家新药筛选中心实验室纯化。
2.2. 化合物2-19的合成
将HATU (4.07 g, 10.71 mmol)和DIPEA (2.30 g, 17.86 mmol)加入2-呋喃甲酸(1.00 g, 8.93 mmol)在DMF (10 mL)中的溶液,在氮气保护下在室温反应1小时。然后将间氨基苯甲酸甲酯(1.48 g, 9.82 mmol)在氮气保护下在室温下添加到溶液中过夜。反应完成后,加入乙酸乙酯稀释,并用1 M HCl溶液和饱和碳酸氢钠水溶液洗涤。最后用饱和氯化钠洗涤多次,浓缩有机相,得到化合物2 (2.1 g, 8.11 mmol, 产率90.8%)。1H NMR (400 MHz, DMSO-d6) δ: 10.40 (s, 1H), 8.42 (t, J = 2.0 Hz, 1H), 8.06~8.02 (m, 1H), 7.96 (dd, J = 1.6, 0.8 Hz, 1H), 7.71~7.67 (m, 1H), 7.50 (t, J = 8.0 Hz, 1H), 7.38 (dd, J = 3.6, 0.8 Hz, 1H), 6.72 (dd, J = 3.6, 1.6 Hz, 1H), 3.87 (s, 3H)。
用类似方法合成化合物3~10。
3-(呋喃-2-甲酰胺基)-2-甲基苯甲酸甲酯(3),收率90.2%;1H NMR (400 MHz, DMSO-d6) δ: 9.97 (s, 1H), 7.93 (d, J = 2.0 Hz, 1H), 7.65 (dd, J = 8.0, 1.6 Hz, 1H), 7.51 (dd, J = 8.0, 1.6 Hz, 1H), 7.37~7.26 (m, 2H), 6.70 (dd, J = 3.6, 1.6 Hz, 1H), 2.33 (s, 3H)。
3-(呋喃-2-甲酰胺基)-4-甲基苯甲酸甲酯(4),收率85.4%;1H NMR (400 MHz, DMSO-d6) δ: 9.89 (s, 1H), 8.01~7.90 (m, 2H), 7.75 (dd, J = 8.0, 2.0 Hz, 1H), 7.42 (d, J = 8.0 Hz, 1H), 7.31 (dd, J = 3.2, 0.8 Hz, 1H), 6.71 (dd, J = 3.6, 2.0 Hz, 1H), 3.85 (s, 3H), 2.30 (s, 3H)。
5-(呋喃-2-羧酰胺基)-2-甲基苯甲酸甲酯(5),收率97.7%;1H NMR (400 MHz, DMSO-d6) δ: 10.29 (s, 1H), 8.26 (d, J = 2.4 Hz, 1H), 7.94 (d, J = 2.0 Hz, 1H), 7.87 (dd, J = 8.4, 2.4 Hz, 1H), 7.34 (d, J = 3.6 Hz, 1H), 7.30 (d, J = 8.4 Hz, 1H), 6.70 (dd, J = 3.6, 1.6 Hz, 1H), 3.84 (s, 4H), 2.48 (s, 4H)。
3-(5-甲基呋喃-2-羧酰胺基)苯甲酸甲酯(6),收率91.1%;1H NMR (400 MHz, DMSO-d6) δ: 10.23 (s, 1H), 8.40 (t, J = 2.0 Hz, 1H), 8.07~8.02 (m, 1H), 7.69~7.65 (m, 1H), 7.48 (t, J = 8.0 Hz, 1H), 7.28 (d, J = 3.6 Hz, 1H), 6.34 (dd, J = 3.6, 1.2 Hz, 1H), 3.87 (s, 3H), 2.39 (s, 3H)。
3-(呋喃-3-甲酰胺基)苯甲酸甲酯(7),收率97.8%;1H NMR (400 MHz, DMSO-d6) δ: 10.13 (s, 1H), 8.41 (s, 1H), 8.35 (t, J = 2.0 Hz, 1H), 8.09~8.00 (m, 1H), 7.81 (t, J = 2.0 Hz, 1H), 7.68 (d, J = 8.0 Hz, 1H), 7.50 (t, J = 8.0 Hz, 1H), 7.01 (d, J = 2.0 Hz, 1H), 3.87 (s, 3H)。
3-(噻吩-2-甲酰胺基)苯甲酸甲酯(8),收率89.1%;1H NMR (400 MHz, DMSO-d6) δ: 10.43 (s, 1H), 8.39 (t, J = 2.0 Hz, 1H), 8.13~8.02 (m, 2H), 7.87 (dd, J = 4.8, 1.2 Hz, 1H), 7.71~7.67 (m, 1H), 7.50 (t, J = 8.0 Hz, 1H), 7.24 (dd, J = 4.8, 3.6 Hz, 1H), 3.87 (s, 3H)。
3-苯甲酰氨基苯甲酸甲酯(9),收率81.8%;1H NMR (400 MHz, DMSO-d6) δ: 10.46 (s, 1H), 8.47 (t, J = 2.0 Hz, 1H), 8.09~8.05 (m, 1H), 8.02~7.96 (m, 2H), 7.72~7.68 (m, 1H), 7.64~7.47 (m, 4H), 3.87 (s, 3H)。
4-(呋喃-2-甲酰胺基)苯甲酸甲酯(10),收率93.2%;1H NMR (400 MHz, DMSO-d6) δ: 10.47 (s, 1H), 7.98~7.89 (m, 5H), 7.40 (dd, J = 3.6, 0.8 Hz, 1H), 6.72 (dd, J = 3.6, 1.6 Hz, 1H), 3.83 (s, 3H)。
将化合物2 (1.00 g, 4.08 mmol)完全溶解在甲醇(50 mL)中,在冰浴下加入氢氧化钠(816 mg, 20.41 mmol)室温下过夜。反应完成后,减压蒸馏除去甲醇后加入水完全溶解,向溶液中滴加1M HCl溶液至弱酸性。抽滤,固体真空干燥得到化合物11 (750 mg, 3.25 mmol, 产率79.5%)。1H NMR (400 MHz, DMSO-d6) δ: 12.93 (s, 1H), 10.36 (s, 1H), 8.38 (t, J = 2.0 Hz, 1H), 8.02~7.98 (m, 1H), 7.95 (d, J = 1.6 Hz, 1H), 7.69~7.65 (m, 1H), 7.46 (t, J = 8.0 Hz, 1H), 7.37 (d, J = 3.6 Hz, 1H), 6.71 (dd, J = 3.6, 1.6 Hz, 1H)。
用类似方法合成化合物12~19。
3-(呋喃-2-甲酰胺基)-2-甲基苯甲酸(12),收率76.6%;1H NMR (400 MHz, DMSO-d6) δ: 12.95 (s, 1H), 9.94 (s, 1H), 7.94~7.92 (m, 1H), 7.65 (dd, J = 7.6, 1.2 Hz, 1H), 7.50~7.42 (m, 1H), 7.34~7.24 (m, 2H), 6.70 (dd, J = 3.6, 1.6 Hz, 1H), 2.35 (s, 3H)。
3-(呋喃-2-甲酰胺基)-4-甲基苯甲酸(13),收率80.1%;1H NMR (400 MHz, DMSO-d6) δ: 12.88 (s, 1H), 9.88 (s, 1H), 7.95~7.91 (m, 2H), 7.73 (dd, J = 8.0, 1.6 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 7.31 (d, J = 3.2 Hz, 1H), 6.71 (dd, J = 3.6, 1.6 Hz, 1H), 2.29 (s, 3H)。
5-(呋喃-2-羧酰胺基)-2-甲基苯甲酸(14),收率88.7%;1H NMR (400 MHz, DMSO-d6) δ: 12.84 (s, 1H), 10.24 (s, 1H), 8.24 (d, J = 2.4 Hz, 1H), 7.94~7.92 (m, 1H), 7.84 (dd, J = 8.0, 2.4 Hz, 1H), 7.34 (d, J = 3.6 Hz, 1H), 7.26 (d, J = 8.4 Hz, 1H), 6.70 (dd, J = 3.6, 1.6 Hz, 1H), 2.48 (s, 4H)。
3-(5-甲基呋喃-2-羧酰胺基)苯甲酸(15),收率66.3%;1H NMR (400 MHz, DMSO-d6) δ: 12.94 (s, 1H), 10.17 (s, 1H), 8.36 (t, J = 2.0 Hz, 1H), 8.02-7.98 (m, 1H), 7.67~7.63 (m, 1H), 7.45 (t, J = 8.0 Hz, 1H), 7.27 (d, J = 3.6 Hz, 1H), 6.34~6.32 (m, 1H), 2.39 (s, 3H)。
3-(呋喃-3-甲酰胺基)苯甲酸(16),收率69.4%;1H NMR (400 MHz, DMSO-d6) δ: 10.11 (s, 1H), 8.40 (s, 1H), 8.31 (s, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.79 (s, 1H), 7.66 (d, J = 7.6 Hz, 1H), 7.47 (t, J = 8.0 Hz, 1H), 7.01 (s, 1H)。.
3-(噻吩-2-甲酰胺基)苯甲酸(17),收率76.8%;1H NMR (400 MHz, DMSO-d6) δ: 12.99 (s, 1H), 10.40 (s, 1H), 8.34 (t, J = 2.0 Hz, 1H), 8.06 (dd, J = 4.0, 1.2 Hz, 1H), 8.02 (dd, J = 8.0, 2.4 Hz, 1H), 7.93~7.84 (m, 1H), 7.74~7.64 (m, 1H), 7.48 (t, J = 8.0 Hz, 1H), 7.24 (dd, J = 5.2, 3.6 Hz, 1H)。
3-苯甲酰氨基苯甲酸(18),收率63.6%;1H NMR (400 MHz, DMSO-d6) δ: 12.97 (s, 1H), 10.42 (s, 1H), 8.43 (d, J = 2.0 Hz, 1H), 8.05~8.03 (m, 1H), 8.01~7.95 (m, 2H), 7.73~7.64 (m, 1H), 7.65~7.43 (m, 4H)。
4-(呋喃-2-甲酰胺基)苯甲酸(19),收率91.4%;1H NMR (400 MHz, DMSO-d6) δ: 12.71 (s, 1H), 8.00~7.86 (m, 5H), 7.40 (d, J = 3.6 Hz, 1H), 6.72 (dd, J = 3.6, 1.6 Hz, 1H)。
3. 结果与讨论
本研究以Na3VO3作为阳性对照,表1中汇总了评价氨基苯甲酸衍生物(2~19)对SHP2PTP的抑制活性数据 [29]。实验结果表明,含有酯基的化合物(2~8)对SHP2的抑制活性明显弱于含羧基的化合物(11~17),这些结果表明,羧基对于抑制酶的活性起到积极的作用。在氨基苯甲酸的苯环上引入甲基(化合物12~14),活性弱于未被取代的化合物11,这些结果说明,在苯环上引入甲基,不利于抑制活性的提高。通过改变R3取代基的结构类型(化合物15~19),化合物15 (含5-甲基呋喃-2-甲酰胺基),,化合物16 (呋喃-3-甲酰胺基)和化合物17 (噻吩-2-甲酰胺基)也显示出与化合物11 (含呋喃-2-甲酰胺基)近似的活性,而化合物18 (含苯甲酰氨基)对SHP2的抑制活性明显下降。这些结果说明酰胺键连接的芳环类型对该类化合物的抑制活性产生一定的影响。将化合物11的3位呋喃-2-甲酰胺基更换到4位上得到化合物19,化合物对SHP2的抑制活性有一定程度的下降。遗憾的是,该化合物对SHP2催化域的抑制活性明显低于阳性对照Na3VO3。

Table 1. SHP2PTP inhibitory activities of compounds 2~19
表1. 化合物2~19对SHP2PTP的抑制活性

a Values tested at 50 μM concentration; b Positive control.
4. 结论
本文为拓展SHP2抑制剂的结构多样性,设计并合成了18个氨基苯甲酸衍生物,其中3-(5-甲基呋喃-2-羧酰胺基)苯甲酸(化合物15)表现了一定的抑制活性,通过构效关系分析,结果表明羧基对于活性有重要影响。同时,在氨基上引入的杂环的类型以及芳环上取代基的类型,对化合物的活性产生一定的影响。该类化合物分子量小,易于衍生化,可以作为活性片段应用于后续的SHP2抑制剂的开发。
基金项目
本文为江苏省自然科学基金BK20190608资助。