1. 引言
茚酮结构较广泛存在于许多具有生物活性的天然产物和药物分子中 [1] [2]。据报道,许多3-氨基-1-茚酮类化合物具有较好的生物活性和医药价值。例如:某些3-(2-四氢萘胺基)-1-茚酮(图1,A)具有稳定细胞及抗过敏的活性 [3] [4];一些2-芳亚甲基-3-氨基-1-茚酮类(图1,B)可能具有双特异磷酸酯酶抑制活性 [5] [6];某类3-氨基-1-茚酮衍生物(图1,C)可作为潜在的AChE抑制剂 [7]。
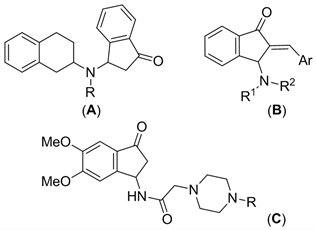
Figure 1. Some biologically active 3-aminoindan-1-ones
图1. 某些具有生物活性的3-氨基-1-茚酮化合物
由于3-氨基-1-茚酮类具有重要应用价值,已相继出现了许多合成报道。合成3-氨基-1-茚酮其中一种较为通用的方法是α-氨基芳乙酸类的分子内傅克酰化反应 [8] [9]。另一类较常见方法是利用3-卤-1-茚酮与胺类的取代反应。例如:Day等报道了采用3-溴-1-茚酮与过量胺反应可制备3-氨基-1-茚酮 [3]。然而该法需要用到3-溴-1-茚酮这样的特定底物,应用范围有限。2004年,Larhed等采用微波促进的钯催化邻溴苯基烯胺羰基化环化反应制备了一系列3-酰氨基-1-茚酮 [10]。2006年,Wu课题组利用1-茚酮与胺类的氮杂Michael加成制备了3-氨基-1-茚酮 [11]。2019年,Wang等报道了邻乙酰基苯甲醛和仲胺经Mannich环化反应制备一系列3-氨基-1-茚酮,产率中等至优秀 [12]。然而,大多数现存相关方法仍然存在许多缺点,例如需预先制备特定结构底物、效率较低、时间较长、操作繁琐、适用范围狭窄等。Wong课题组采用邻乙炔基苯甲醛与仲胺在无催化剂条件下经烯胺化/环化和硅胶色谱柱分离(伴随水解),获得了一系列3-氨基-1-茚酮(58%~86%, 24~48 h) [13]。然而,探寻更高效和快速的一锅制备这类化合物的方法仍然显得很有必要。
本文提供一种以廉价Bronsted酸作为催化剂,邻乙炔基芳醛和仲胺经烯胺化/环化/水解的串联反应,便捷、高效地合成3-氨基-1-茚酮的方法。
2. 实验部分
2.1. 仪器与试剂
仪器:WRS-1B 数字熔点仪(温度计未经校正);Bruker Avance 500型核磁共振波谱仪(CDCl3或DMSO为溶剂,TMS为内标);有机反应采用薄层硅胶板(TLC)跟踪监测;柱色谱分离采用硅胶(GF254, 200-300目)为固定相,石油醚–乙酸乙酯混合溶剂为洗脱剂。
试剂:邻溴苯甲醛;4-甲基-2-溴苯甲醛;5-甲基-2-溴苯甲醛;6-甲基-2-溴苯甲醛;4-甲氧基-2-溴苯甲醛;5-甲氧基-2-溴苯甲醛;3-甲氧基-2-溴苯甲醛;5-氯-2-溴苯甲醛;4-氟-2-溴苯甲醛;5-氟-2-溴苯甲醛;6-溴苯并[d][1,3]二噁烷-5-甲醛;双(三苯基膦)氯化钯;三甲基硅基乙炔;四氢异喹啉(THIQ);苯甲酸;对甲苯磺酸;三氟甲磺酸;乙酸;2-乙基己酸(2-EHA);甲苯;四氢呋喃;乙醇;DMF等。这些试剂均为市售分析纯。本文所涉及邻乙炔基芳醛类底物,采用已报道相关文献合成方法 [14] [15] [16],由邻溴芳醛类与三甲基硅基乙炔经钯催化Sonogashira偶联制备而得,氢谱数据与已报道文献相关化合物数据吻合。
2.2. 3-氨基-1-茚酮的一般合成步骤及产物结构分析
在5毫升的试管反应器里,加入环状仲胺2 (0.875 mmol, 3.5 eq)、邻乙炔基芳醛1 (0.25 mmol)、苯甲酸(0.0375 mmol, 15 mol%)、甲苯(1.0 mL),和水(0.05 mmol, 20 mol%),于室温下磁力预搅拌0.5 h,之后加热到50℃搅拌反应。TLC检测,待反应完成后,减压浓缩,残留物经过柱层析(硅胶150~200目;石油醚:乙酸乙酯 = 6:1~12:1),获得相应目标产物3 (反应式如图2所示)。
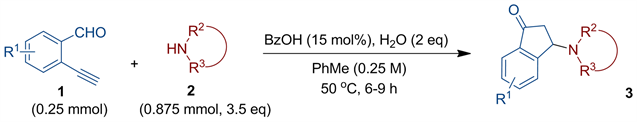
Figure 2. Bronsted acid-catalyzed one-pot synthesis of 3-aminoindan-1-ones
图2. 3-氨基-1-茚酮的酸催化一锅合成
目标产物3的表征数据如下:
3-(3,4-Dihydroisoquinolin-2(1H)-yl)-2,3-dihydro-1H-inden-1-one, 3a [13]: Yield 90%; Brown vicious oil; 1H NMR (500 MHz, CDCl3) δ 7.79 (dd, J = 17.5, 7.7 Hz, 2H), 7.66 (td, J = 7.6, 1.2 Hz, 1H), 7.48 (t, J = 7.4 Hz, 1H), 7.18~7.05 (m, 3H), 7.01~6.96 (m, 1H), 4.80 (dd, J = 7.0, 3.5 Hz, 1H), 3.81 and 3.63 (ABq, J = 14.6 Hz, 2H), 2.96~2.86 (m, 2H), 2.85~2.80 (m, 1H), 2.76~2.69 (m, 2H), 2.67~2.62 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 204.5, 154.4, 137.7, 135.1, 134.7, 134.4, 129.00, 128.97, 126.9, 126.7, 126.3, 125.8, 123.4, 62.6, 52.0, 45.9, 36.2, 29.8; HRMS (ESI-TOF) calcd for C18H18NO (M+H)+ 264.1383, found 264.1387.
3-(3,4-Dihydroisoquinolin-2(1H)-yl)-6-methyl-2,3-dihydro-1H-inden-1-one, 3b [13]: Yield 77%; Brown vicious oil; 1H NMR (500 MHz, CDCl3) δ 7.63 (d, J = 7.8 Hz, 1H), 7.60 (s, 1H), 7.48 (dd, J = 7.8, 1.1 Hz, 1H), 7.16~7.06 (m, 3H), 7.00~6.95 (m, 1H), 4.75 (dd, J = 7.0, 3.4 Hz, 1H), 3.79 and 3.61 (ABd, J = 14.6 Hz, 2H), 2.94~2.85 (m, 2H), 2.81 (dd, J = 18.9, 3.5 Hz, 1H), 2.75~2.68 (m, 2H), 2.66~2.61 (m, 1H), 2.44 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 204.7, 151.8, 139.1, 137.9, 136.3, 134.8, 134.5, 128.9, 126.7, 126.6, 126.3, 125.8, 123.3, 62.3, 51.9, 45.9, 36.6, 29.8, 21.3; HRMS (ESI-TOF) calcd for C19H20NO (M+H)+ 278.1539, found 278.1541.
3-(3,4-Dihydroisoquinolin-2(1H)-yl)-5-methyl-2,3-dihydro-1H-inden-1-one, 3c: Yield 86%; Yellow solid; m.p. 160.5˚C~162.0˚C (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 7.9 Hz, 1H), 7.56 (s, 1H), 7.28 (dd, J = 7.9, 0.6 Hz, 1H), 7.19~7.06 (m, 3H), 6.98 (d, J = 6.7 Hz, 1H), 4.75 (dd, J = 7.0, 3.4 Hz, 1H), 3.79 and 3.61 (ABq, J = 14.6 Hz, 2H), 2.90 (t, J = 6.3 Hz, 2H), 2.82 (dd, J = 18.8, 3.5 Hz, 1H), 2.76~2.63 (m, 3H), 2.46 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 204.1, 154.9, 146.3, 135.5, 134.8, 134.4, 130.3, 129.0, 127.1, 126.7, 126.3, 125.8, 123.2, 62.5, 51.8, 46.1, 36.2, 29.8, 22.3; C19H20NO (M+H)+ 278.1539, found 278.1532.
3-(3,4-Dihydroisoquinolin-2(1H)-yl)-4-methyl-2,3-dihydro-1H-inden-1-one, 3d: Yield 81%; Yellow solid; m.p. 144.5˚C~146.2˚C (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.64 (d, J = 7.5 Hz, 1H), 7.48~7.41 (m, 1H), 7.38 (t, J = 7.4 Hz, 1H), 7.18~7.05 (m, 3H), 7.02~6.95 (m, 1H), 4.84 (dd, J = 7.2, 2.4 Hz, 1H), 3.76 and 3.53 (ABq, J = 14.7 Hz, 2H), 2.88 (dd, J = 19.1, 2.5 Hz, 1H), 2.85~2.78 (m, 2H), 2.69~2.58 (m, 2H), 2.57~2.53 (m, 1H), 2.52 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 205.4, 152.2, 138.5, 137.8, 136.6, 135.1, 134.7, 129.0, 128.8, 126.7, 126.1, 125.7, 120.6, 62.1, 51.6, 45.2, 34.6, 29.8, 18.3 cm-1; C19H20NO (M+H)+ 278.1539, found 278.1545.
3-(3,4-Dihydroisoquinolin-2(1H)-yl)-6-methoxy-2,3-dihydro-1H-inden-1-one, 3e [12]: Yield 85%; Yellow solid; m.p. 111.8˚C~113.3˚C (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.63 (d, J = 8.2 Hz, 1H), 7.26~7.20 (m, 2H), 7.15~7.06 (m, 3H), 7.00~6.96 (m, 1H), 4.73 (dd, J = 6.8, 3.2 Hz, 1H), 3.86 (s, 3H), 3.78 and 3.60 (ABq, J = 14.6 Hz, 2H), 2.92~2.85 (m, 2H), 2.83 (dd, J = 18.9, 3.2 Hz, 1H), 2.75~2.67 (m, 2H), 2.66~2.61 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 204.5, 160.6, 147.2, 139.0, 134.8, 134.5, 128.9, 127.7, 126.7, 126.3, 125.8, 124.5, 104.5, 62.0, 55.8, 51.9, 45.8, 36.8, 29.8; IR v 3321.8, 1703.0, 1650.2, 1488.6; HRMS (ESI-TOF) calcd for C19H20NO2 (M+H)+ 294.1489, found 294.1493.
3-(3,4-Dihydroisoquinolin-2(1H)-yl)-5-methoxy-2,3-dihydro-1H-inden-1-one, 3f: Yield 88%; Yellow solid; m.p. 158.6˚C~160.5˚C (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.72 (d, J = 8.5 Hz, 1H), 7.19 (s, 1H), 7.16~7.10 (m, 3H), 7.01~6.98 (m, 2H), 4.73 (dd, J = 7.0, 3.5 Hz, 1H), 3.88 (s, 3H), 3.81 and 3.66 (ABq, J = 14.6 Hz, 2H), 2.94~2.87 (m, 2H), 2.81 (dd, J = 18.7, 3.6 Hz, 1H), 2.72~ 2.64 (m, 3H). 13C NMR (125 MHz, CDCl3) δ 202.7, 165.8, 157.9, 134.7, 134.4, 131.0, 129.0, 126.8, 126.4, 125.8, 125.1, 117.5, 109.3, 62.5, 56.0, 52.1, 45.7, 35.9, 29.8; HRMS (ESI-TOF) calcd for C19H20NO2 (M+H)+ 294.1489, found 294.1481.
3-(3,4-Dihydroisoquinolin-2(1H)-yl)-7-methoxy-2,3-dihydro-1H-inden-1-one, 3g: Yield 88%; Yellow vicious oil; 1H NMR (500 MHz, CDCl3) δ 7.58 (t, J = 7.9 Hz, 1H), 7.30 (d, J = 7.6 Hz, 1H), 7.18~7.07 (m, 3H), 6.99~6.93 (m, 1H), 6.88 (d, J = 8.2 Hz, 1H), 4.70 (dd, J = 7.2, 3.6 Hz, 1H), 3.97 (s, 3H), 3.78 and 3.60 (ABq, J = 14.6 Hz, 2H), 2.93~2.84 (m, 2H), 2.80 (dd, J = 18.6, 3.6 Hz, 1H), 2.74~2.58 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 202.3, 157.8, 157.1, 136.7, 134.8, 134.4, 128.9, 127.5, 126.7, 126.2, 125.7, 118.5, 110.3, 62.0, 56.0, 51.8, 45.9, 36.7, 29.8; HRMS (ESI-TOF) calcd for C19H20NO2 (M+H)+ 294.1489, found 294.1478.
5-Chloro-3-(3,4-dihydroisoquinolin-2(1H)-yl)-2,3-dihydro-1H-inden-1-one, 3h [12]: Yield 90%; Yellow solid; m.p. 114.5˚C~116.3˚C (EtOAc); 1H NMR (500 MHz, CDCl3) δ 1H NMR (500 MHz, CDCl3) δ 7.77 (s, 1H), 7.72 (d, J = 8.2 Hz, 1H), 7.44 (ddd, J = 8.1, 1.8, 0.5 Hz, 1H), 7.18~7.08 (m, 3H), 7.01~6.97 (m, 1H), 4.76 (dd, J = 7.1, 3.6 Hz, 1H), 3.80 and 3.62 (ABq, J = 14.5 Hz, 2H), 2.95~2.88 (m, 2H), 2.84 (dd, J = 19.0, 3.7 Hz, 1H), 2.77~2.70 (m, 2H), 2.67~2.62 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 202.9, 156.1, 141.8, 136.0, 134.5, 134.3, 129.8, 129.0, 127.0, 126.7, 126.4, 125.9, 124.6, 62.3, 51.9, 46.0, 36.1, 29.8; HRMS (ESI-TOF) calcd for C18H17ClNO (M+H)+ 298.0992, found 298.0995.
3-(3,4-Dihydroisoquinolin-2(1H)-yl)-6-fluoro-2,3-dihydro-1H-inden-1-one, 3i: Yield 91%; Yellow solid; m.p. 61.2˚C~63.0˚C (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.73 (dd, J = 8.4, 4.6 Hz, 1H), 7.43 (dd, J = 7.5, 2.5 Hz, 1H), 7.39~7.33 (m, 1H), 7.20~7.07 (m, 3H), 7.02~6.96 (m, 1H), 4.76 (dd, J = 6.9, 3.3 Hz, 1H), 3.79 and 3.61 (ABq, J = 14.5 Hz, 2H), 2.92~2.83 (m, 3H), 2.78~2.68 (m, 2H), 2.65~2.60 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 203.4, 163.3 (d, J = 249.7 Hz), 150.0, 139.4 (d, J = 7.4 Hz), 134.6, 134.4, 129.0, 128.4 (d, J = 8.2 Hz), 126.7, 126.4, 125.9, 122.8 (d, J = 23.7 Hz), 109.2 (d, J = 21.9 Hz), 62.1, 52.0, 45.8, 36.5, 29.7; HRMS (ESI-TOF) calcd for C18H17FNO (M+H)+ 282.1289, found 282.1286.
3-(3,4-Dihydroisoquinolin-2(1H)-yl)-5-fluoro-2,3-dihydro-1H-inden-1-one, 3j [12]: Yield 88%; Yellow solid; m.p. 129.3˚C~130.9˚C (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.81 (dd, J = 8.5, 5.2 Hz, 1H), 7.44 (d, J = 7.9 Hz, 1H), 7.22~7.06 (m, 4H), 7.03~6.96 (m, 1H), 4.77 (dd, J = 7.0, 3.6 Hz, 1H), 3.82 and 3.65 (ABq, J = 14.5 Hz, 2H), 2.96~2.82 (m, 3H), 2.78~2.70 (m, 2H), 2.68~2.63 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 202.5, 167.6 (d, J = 257.1 Hz), 134.5, 134.3, 134.0, 129.0, 126.7, 126.4, 125.9, 125.8, 125.8 (d, J = 10.3 Hz), 117.4 (d, J = 24.1 Hz), 113.4 (d, J = 22.4 Hz), 62.3, 52.0, 45.9, 36.2, 29.7; HRMS (ESI-TOF) calcd for C18H17FNO (M+H)+ 282.1289, found 282.1297.
7-(3,4-Dihydroisoquinolin-2(1H)-yl)-6,7-dihydro-5H-indeno[5,6-d][1,3]dioxol-5-one, 3k: Yield 90%; Yellow solid; m.p. 146.8˚C~148.7˚C (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.18~7.07 (m, 5H), 7.00~6.96 (m, 1H), 6.09 (d, J = 4.4 Hz, 2H), 4.64 (dd, J = 6.8, 3.2 Hz, 1H), 3.77 and 3.61 (ABq, J = 14.6 Hz, 2H), 2.91~2.85 (m, 2H), 2.79 (d, J = 18.8, 3.2 Hz, 1H), 2.71~2.62 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 202.3, 154.6, 152.2, 149.5, 134.8, 134.5, 132.5, 129.0, 126.7, 126.3, 125.8, 105.8, 102.5, 101.8, 62.2, 51.9, 45.7, 36.1, 29.8; HRMS (ESI-TOF) calcd for C19H18NO3 (M+H)+ 308.1281, found 308.1277.
3-(5-Methyl-3,4-dihydroisoquinolin-2(1H)-yl)-2,3-dihydro-1H-inden-1-one, 3l: Yield 88%; Yellow vicious oil; 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 7.7 Hz, 1H), 7.78~7.72 (m, 1H), 7.66 (dt, J = 7.6, 1.2 Hz, 1H), 7.51~7.45 (m, 1H), 7.09~6.97 (m, 2H), 6.85 (d, J = 7.3 Hz, 1H), 4.80 (dd, J = 7.1, 3.5 Hz, 1H), 3.79 and 3.61 (ABq, J = 14.4 Hz, 2H), 2.84 (dd, J = 18.9, 3.5 Hz, 1H), 2.77~2.65 (m, 5H), 2.22 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 204.6, 154.4, 137.7, 136.6, 135.0, 134.7, 133.0, 128.9, 127.7, 126.9, 125.6, 124.4, 123.4, 62.5, 52.4, 46.1, 36.1, 27.6, 19.2; HRMS (ESI-TOF) calcd for C19H20NO (M+H)+ 278.1539, found 278.1548.
3-(7-Methoxy-3,4-dihydroisoquinolin-2(1H)-yl)-2,3-dihydro-1H-inden-1-one, 3m: Yield 85%; Yellow vicious oil; 1H NMR (500 MHz, CDCl3) δ 7.79 (d, J = 7.7 Hz, 1H), 7.76 (dd, J = 7.7, 0.8 Hz, 1H), 7.67~7.63 (m, 1H), 7.49~7.45 (m, 1H), 6.90 (d, J = 8.4 Hz, 1H), 6.69 (dd, J = 8.4, 2.7 Hz, 1H), 6.63 (d, J = 2.7 Hz, 1H), 4.78 (dd, J = 7.1, 3.5 Hz, 1H), 3.76 (s, 3H), 3.75 and 3.56 (ABq, J = 14.2 Hz, 2H), 2.89~2.83 (m, 2H), 2.82~2.77 (m, 1H), 2.74~2.65 (m, 2H), 2.64~2.59 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 204.5, 158.1, 154.4, 137.6, 135.5, 135.0, 128.9, 127.6, 126.9, 126.8, 123.3, 113.5, 112.2, 62.6, 55.4, 51.5, 45.7, 36.2, 30.1; HRMS (ESI-TOF) calcd for C19H20NO2 (M+H)+ 294.1489, found 294.1485.
3-(6,7-Dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)-2,3-dihydro-1H-inden-1-one, 3n [12]: Yield 86%; Yellow vicious oil; 1H NMR (500 MHz, CDCl3) δ 7.79 (d, J = 7.7 Hz, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.66 (dd, J = 7.6, 1.1 Hz, 1H), 7.47 (t, J = 7.4 Hz, 1H), 6.59 (s, 1H), 6.48 (s, 1H), 4.79 (dd, J = 7.0, 3.4 Hz, 1H), 3.84 (s, 3H), 3.80 (s, 3H), 3.72 and 3.53 (ABq, J = 14.2 Hz, 2H), 2.85~2.78 (m, 3H), 2.74 (d, J = 7.1 Hz, 1H), 2.71~2.68 (m, 1H), 2.66~2.61 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 204.6, 154.4, 147.7, 147.4, 137.7, 135.0, 129.0, 126.9, 126.5, 126.3, 123.4, 111.6, 109.6, 62.6, 56.1, 56.0, 51.4, 46.2, 36.3, 29.4; HRMS (ESI-TOF) calcd for C20H22NO3 (M+H)+ 324.1594, found 324.1582.
3-(7,8-Dihydro-[1,3]dioxolo[4,5-g]isoquinolin-6(5H)-yl)-2,3-dihydro-1H-inden-1-one, 3o: Yield 87%; Yellow solid; m.p. 174.2˚C~175.6˚C (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.82~7.72 (m, 2H), 7.68~7.64 (m, 1H), 7.47 (t, J = 7.5 Hz, 1H), 6.56 (s, 1H), 6.45 (s, 1H), 5.88 (dd, J = 2.8, 1.4 Hz, 2H), 4.78 (dd, J = 6.5, 3.2 Hz, 1H), 3.71 and 3.53 (ABq, J = 14.3 Hz, 2H), 2.84~2.75 (m, 3H), 2.72 (dd, J = 18.9, 7.0 Hz, 1H), 2.68~2.58 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 204.4, 154.2, 146.3, 145.9, 137.7, 135.1, 129.0, 127.4, 127.3, 126.9, 123.4, 108.7, 106.6, 100.8, 62.5, 52.1, 45.8, 36.3, 29.8; HRMS (ESI-TOF) calcd for C19H18NO3 (M+H)+ 308.1281, found 308.1287.
3-(Pyrrolidin-1-yl)-2,3-dihydro-1H-inden-1-one, 3p [13]: Yield 97%; Brown vicious oil; 1H NMR (500 MHz, CDCl3) δ 7.76~7.73 (m, 1H), 7.71~7.68 (m, 1H), 7.62 (td, J = 7.6, 1.2 Hz, 1H), 7.44~7.40 (m, 1H), 4.62 (t, J = 4.9 Hz, 1H), 2.72~2.70 (m, 2H), 2.65~2.60 (m, 2H), 2.53~2.48 (m, 2H), 1.80~1.75 (m, 4H); 13C NMR (125 MHz, CDCl3) δ 204.8, 155.1, 137.3, 134.7, 128.7, 127.1, 123.4, 59.3, 49.6, 38.5, 23.6; HRMS (ESI-TOF) calcd for C13H16NO (M+H)+ 202.1226, found 202.1229.
3-(Azepan-1-yl)-2,3-dihydro-1H-inden-1-one, 3q [13]: Yield 83%; Yellow vicious oil; 1H NMR (500 MHz, CDCl3) δ 7.74 (d, J = 7.8 Hz, 2H), 7.63 (t, J = 7.5 Hz, 1H), 7.41 (t, J = 7.4 Hz, 1H), 4.58 (dd, J = 5.2, 3.7 Hz, 1H), 2.73 (dd, J = 18.8, 6.9 Hz, 1H), 2.65~2.54 (m, 3H), 2.53~2.44 (m, 2H), 1.70~1.51 (m, 8H); 13C NMR (125 MHz, CDCl3) δ 205.3, 156.1, 137.7, 134.8, 128.6, 126.7, 123.2, 64.2, 52.0, 37.9, 29.8, 26.9; HRMS (ESI-TOF) calcd for C15H20NO (M+H)+ 230.1539, found 230.1542.
3-(4,5-Dihydro-1H-benzo[c]azepin-2(3H)-yl)-2,3-dihydro-1H-inden-1-one, 3r: Yield 89%; Yellow vicious oil; 1H NMR (500 MHz, CDCl3) δ 7.80 (d, J = 7.7 Hz, 1H), 7.69 (d, J = 7.6 Hz, 1H) 7.66~7.63 (m, 1H), 7.49~7.45 (m, 1H), 7.17~7.10 (m, 2H), 7.07 (dt, J = 7.0, 2.1 Hz, 1H), 6.80 (d, J = 7.3 Hz, 1H), 4.69~4.66 (m, 1H), 3.60 and 3.45 (ABq, J = 13.7 Hz, 2H), 3.13~3.08 (m, 1H), 2.95~2.83 (m, 3H), 2.77~2.70 (m, 2H), 1.90~1.81 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 204.7, 155.2, 142.6, 140.2, 137.7, 134.9, 129.1, 128.8, 128.6, 127.2, 126.9, 126.3, 123.3, 65.0, 58.0, 56.8, 37.9, 35.2, 29.3; HRMS (ESI-TOF) calcd for C19H20NO (M+H)+ 278.1539, found 278.1532.
3-(3,4-Dihydro-1H-pyrido[3,4-b]indol-2(9H)-yl)-2,3-dihydro-1H-inden-1-one, 3s [12]: Yield 81%; Yellow vicious oil; 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 7.4 Hz 2H), 7.78 (d, J = 0.6 Hz 1H), 7.69~7.64 (m, 1H), 7.51~7.44 (m, 2H), 7.28 (dd, J = 7.9, 0.9 Hz, 1H), 7.29~7.26 (m, 2H), 4.84 (dd, J = 7.0, 3.6 Hz, 1H), 3.76 and 3.61 (ABq, J = 14.1 Hz, 2H), 3.64~3.59 (m, 5H), 2.76 (dd, J = 19.0, 7.1 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 204.4, 154.3, 137.6, 136.2, 135.2, 131.6, 129.1, 127.3, 127.0, 123.5, 121.6, 119.6, 118.1, 110.9, 108.7, 62.7, 47.0, 46.3, 36.7, 22.2; HRMS (ESI-TOF) calcd for C20H19N2O (M+H)+ 303.1492, found 303.1497.
副产物4a的表征数据如下:
By-product, 4a [17]: Yield 29%; White solid; m.p. 38.7-40.3 °C (EtOAc); 1H NMR (500 MHz, CDCl3) δ 10.22 (s, 1H), 7.88~7.86 (m, 1H), 7.68~7.63 (m, 2H), 2.65 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 201.1, 192.3, 140.7, 136.3, 133.1, 132.0, 129.8, 128.6, 28.9.
2.3. 放大反应研究
在250毫升的烧瓶里,加入THIQ 2a (70 mmol, 3.5 eq)、邻乙炔基苯甲醛1a (20 mmol)、苯甲酸(3 mmol, 15 mol%)、甲苯(80 mL),和水(4 mmol, 20 mol%),于室温下磁力预搅拌0.5 h,之后加热到50℃搅拌反应。TLC检测,待反应完成后,减压浓缩,残留物经过柱层析(硅胶150~200目;石油醚:乙酸乙酯 = 10:1),获得相应目标产物3a。
3. 结果与讨论
3.1. 反应条件的优化
本文采用廉价的质子酸作为催化剂,考察了在不同条件下的反应情况(表1)。如表1所示,在苯甲酸(20 mol%)催化下,邻乙炔基苯甲醛(0.25 mmol)与四氢异喹啉(THIQ) (0.5 mmol)在甲苯(1 mL)中的反应作为模板反应进行条件筛选。在40℃反应12个小时,目标产物3a产率仅为36%,伴有18%的副产物4a生成(表1,entry 1),提高反应温度为50℃,经反应8小时,目标产物3a产率稍有所提升(表1,entry 2)。再提高温度至60℃,经反应6小时,目标产物产率反而略有下降,而副产物比例略有提高(表1,entry 3)。考虑到加水可能有助于烯胺中间体水解,我们尝试在体系中加入水,发现引入水的量对反应有较明显影响(表1,entries 4-6),结果显示2当量水的加入对于该反应最为有利(表1,entry 5)。之后,还考察体系浓度的影响,发现增加或减少溶剂的量,产率均略有下降(表1,entries 7-8)。对于多种质子酸催化剂的筛选实验表明,苯甲酸确实为所试催化剂的最佳之选(表1,entry 5对比entries 9-12)。我们还考察了催化剂用量对反应的影响,发现15 mol%的苯甲酸效果最好(表1,entry 14对比entries 5, 13与15)。实验还表明,四氢异喹啉的当量对反应存在重要影响(表1,entries 14与16-18),并且3.5当量四氢异喹啉的反应产率可达90% (表1,entry 17)。对于其它溶剂的筛选实验结果证实,甲苯为所试溶剂中最佳的(entry 17对比entries 19-21)。综上所述,该反应最佳的条件为:胺(3.5当量),苯甲酸(15 mol%),水(2当量),甲苯(0.25M浓度),反应温度50℃ (表1,entry 17)。


Table 1. Optimization of the domino reactiona
表1. 串联反应条件的优化a
a除非另有说明,将邻乙炔基苯甲醛1a (0.25 mmol)、四氢异喹啉2a (指定量)、酸催化剂(指定量),H2O (指定量)和溶剂(1 mL)的混合物在指定温度下搅拌反应至TLC检测反应完全。b分离产率。c溶剂2 mL。d溶剂0.5 mL。
3.2. 反应底物的拓展
在优化的条件(表1,entry 17所示条件)下,考察了邻乙炔基芳醛中芳环上取代基电子效应对该反应的影响(图3)。从图3中可以看出,1) 反应效率较高,所有反应在7~9 h内均可以顺利反应完全,收率为81%~91%;2) 反应适用范围较广。无论苯环上各个位置带有供电子基团(4-CH3, 5-Me, 6-Me, 3-OMe, 4-OMe, 5-OMe),还是带有吸电子基团(5-Cl, 4-F, 5-F)的邻乙炔基苯甲醛,在该反应体系中均可以获得较为令人满意的结果(3b-3j),收率最高可以达到91%;3) 某个带杂环的邻乙炔基芳醛,也可顺利与THIQ反应得到良好产率的预期产物(3k)。4) 芳环上位阻效应稍有一定影响,但并不是太明显,如芳环邻位分别存在甲基(3d)或甲氧基(3g)时,收率相对略低一点,但也能达到良好(81%~82%)。遗憾的是,对于非端炔基底物如邻(苯乙炔基)苯甲醛与THIQ的反应也进行了尝试,该反应体系较杂,且未分离到预期产物。
在完成对各种取代的邻乙炔基苯甲醛底物的考察之后,我们进一步研究了各种结构环状仲胺在该类反应中的适用性。如图4所示,发现其它许多脂肪族环状仲胺也均能很好地参与这一串联环化反应,且其中有的反应更为迅速(5~9 h)。对于一系列取代的四氢异喹啉相关反应考察的结果表明,这些取代四氢异喹啉均能与邻乙炔基苯甲醛顺利反应得到良好产率的预期产物(3l-3o)。将该方法应用于非六元环状仲胺的串联反应实验也获得了成功(3p-3r)。其中,邻乙炔基苯甲醛与四氢吡咯的反应产率达到了97%,时间也缩短至5 h (3p)。将胺换成七元环状的氮杂䓬或苯并氮杂䓬,反应也可顺利进行,分别以较好的产率得到预期产物(3q-3r)。此后,我们还尝试了邻乙炔基苯甲醛与含三并杂环胺1,2,3,4-四氢-9H-吡啶[3,4-b]并吲哚的反应,并发现该反应也可以顺利地获得相应产物(3s)。期间,我们也曾尝试过采用链状仲胺如二苄胺的反应,遗憾的是,在该体系中无法生成预期的环化产物。
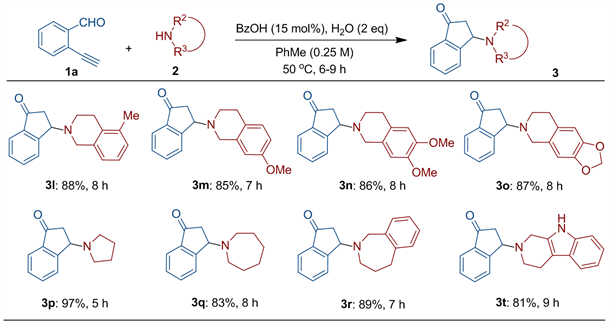
Figure 4. Scope of the cyclic secondary amines
图4. 环状仲胺的拓展
3.3. 放大反应研究
最后,我们还对最初的反应进行了放大量的研究。如下方程式所示,将反应放大80倍(即底物醛20 mmol投料量),也可以一次获得约4.3克目标产物,收率为82% (图5),尽管反应时间需适当延长。
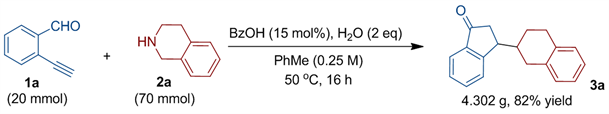
Figure 5. The scalability of domino synthesis of a 3-aminoindan-1-one
图5. 3-氨基-1-茚酮放大量串联合成研究
3.4. 反应机理推测
基于上述实验结果及相关文献报道 [12] [13],我们对反应的可能机理进行了推测。如图6所示,在酸催化条件下,邻乙炔基芳醛羰基质子化得到活化;然后胺进攻活化的羰基,并发生质子转移,形成质子化α-羟基胺中间体I;之后脱水消除,生成亚铵离子中间体II;在质子促进下,炔键受到胺进攻,产生烯胺结构,形成了中间体III;后者发生分子内烯胺α-位对亚铵离子的进攻,生成亚铵离子中间体IV,最后水解生成产物(Path a)。也可能II中的炔键在亚铵离子亲电性活化下,直接接受胺的进攻,发生协同型的加成/环化过程(伴随质子转移和双键异构化),生成亚铵离子中间体IV,最后水解生成产物(Path b)。这里,质子酸可能起到了对醛羰基和炔键的双重亲电性活化作用,并且还有利于最后中间体的互变异构和水解。
4. 结论
在甲苯溶剂中,采用廉价易得的苯甲酸为催化剂,以易得的邻乙炔基芳香醛与脂肪族环状仲胺于较为温和的条件下经烯胺化/环化/水解的串联反应,高效、便捷地得到了各种取代的3-氨基-3-茚酮类化合物。无论是带有吸电子基还是带有推电子基的邻乙炔基芳甲醛均可有效地参与该串联反应;带有各种取代的苯并环状仲胺及单环仲胺亦能顺利地与邻乙炔基苯甲醛反应。在该反应中,质子酸(BzOH)可能起到了活化羰基和炔键的双重作用。所得相关茚酮类化合物具有潜在的生物活性和医药价值。相对于目前已报道的相关化合物合成方法,该方法具有这些优点:1) 产率优良(81%~97%),时间相对较短(5~9 h);2) 反应可放大,在20 mmol规模时收率仍可达到良好;3) 一锅法操作便捷;4) 官能团兼容性佳,底物适用范围较广。此外,反应规模可以放大至一次性合成4.3克以上的产物,收率良好,操作简单。该方法为3-氨基-3-茚酮类化合物的制备提供了一条高效、便捷和实用的新途径。
致谢
感谢浙江省自然科学基金项目(LY21B020005)资助。
NOTES
*通讯作者。