摘要: 通过四磺酸镍酞菁(镍4,4’,4’-四磺基酞菁,NiS4Pc)与一系列阳离子的水相反应发现,NiS4Pc可与Pb2+形成不溶性化合物,而多种阴离子可将其溶解。进一步发现,酸性物质(酸抑制剂)的存在可以抑制阴离子对该化合物的溶解作用。对酸抑制剂的筛选获得了重要的发现:在低浓度柠檬酸存在下,绝大多数阴离子对沉淀的溶解作用被完全或显著抑制,只有P
3O
5-10仍然可以溶解沉淀化合物NiS4Pc-Pb(II),使NiS4Pc被释放至溶液中,溶液相显示出NiS4Pc Pb(II)的特征颜色和吸收光谱,从而为
P3O5-10提供了高特异性的光学探针。溶液的颜色和吸收强度可长期保持稳定,表明这一光学探针具有显著的稳定性。基于这一发现,本研究建立了一种新的光度分析法,用于高特异性定量检测水相中的
P3O5-10。对实验参数进行了优化,在最佳条件下,吸光度差(ΔA621)与
P3O5-10浓度在3.075.0 µg/mL范围内表现出良好的线性相关。回归方程为ΔA621 = 0.014C − 0.057,r = 0.9984。检测限为2.0 µg/mL。与传统的
P3O5-10检测方法相比,该方法具有特异性高、稳定性好、操作简便、快速等特点,具有很强的应用前景。此法还可用于目视化检测,这对于现场或现场分析特别有益。本研究提出了阴离子检测新原理,开拓了金属酞菁化合物在分析科学中的新应用。
Abstract:
A series of reactions between cationic ions and nickel tetrasulfonated phthalocyanine (nickel 4,4’,4’,4’-tetrasulfophthalocyanine, NiS4Pc) were performed and demonstrated that water-soluble NiS4Pc can complex with Pb2+ to form an insoluble compound, many acid anions can dissolve this compound. However, the presence of acidic materials (acid inhibitors) can inhibit the dissolution of this compound by acid anions. Screening of acid inhibitors led to an important finding. In the presence of a low concentration of citric acid, the precipitate dissolution by most anions was completely or significantly inhibited; only P3O5-10 could still dissolve the precipitate compound, NiS4Pc- Pb(II), to release NiS4Pc. The solution phase shows the characteristic color and absorption spectroscopy of NiS4Pc-Pb(II), thus providing a specific optical probe for P3O5-10. In addition, the color and absorption intensity of the solution remained stable for a long time (more than three months), indicating significant stability of the optical probe. Based on this finding, a new spectrophotometric technique has been established for detecting and quantifying aqueous P3O5-10 with high specificity. Furthermore, a new model for the visual (naked eye) detection of P3O5-10 has been developed. The experimental parameters have been optimized. Under the optimal conditions, the absorbance difference (ΔA621) shows an excellent linear correlation with the P3O5-10 concentration (C, µg/mL) ranging from 3.0~75.0 µg/mL. The linear regression equation is ΔA621 = 0.014C − 0.057, with r = 0.9984. The limit of detection was 2.0 µg/mL. Compared to traditional P3O5-10 detection methods, this method exhibits high specificity, excellent stability, easy and fast operation and thus strong application potential. This method can provide visual detection, which will be particularly beneficial for in situ or field analyses. In this study, the preparation of metal phthalocyanine insoluble compound optical probe for highly selective determination of P3O5-10 is universal in principle. It is expected that more optical probes could be constructed based on this principle and applied to the detection of other sorts of anions.
1. 引言
多聚磷酸盐是常用的添加剂,广泛应用于预处理食品 [1] [2] [3] 、日用洗涤产品 [4] [5] [6] 。多聚磷酸盐是铁、锰等金属离子的螯合剂,常用作饮用水的软化剂以抑制水垢的形成 [7] [8] 。人体磷酸盐的摄入量随着生活水平的提高迅速增加 [9] [10] [11] [12] ,而过量磷酸盐的摄入对人体健康是不利的 [13] [14] [15] 。文献报道显示人体内高磷酸盐环境与心血管疾病及肾脏疾病有密切关系 [16] [17] [18] ;大量含磷物质进入水环境中,引起水体富营养化,导致环境生态失衡 [19] [20] 。
以往的多聚磷酸盐的检测技术通常只测定总磷量而难以实现各种磷酸盐型体的特异性检测,欧洲食品安全局因此鼓励开发更具体的(多)磷酸盐分析方法 [21] 。建立简单、快速、直观的多聚磷酸根离子检测新方法具有重要的现实意义。
四磺基镍酞菁(NiS4Pc)是一种理化与光学性质均十分稳定的水溶性金属酞菁化合物。本研究通过筛选实验发现,水溶液中NiS4Pc可与Pb2+离子形成难溶性复合物,进一步发现,多种酸根离子可溶解这一沉淀。有趣的是,在低浓度柠檬酸的存在下,绝大多数阴离子对沉淀的溶解作用几乎被完全抑制,只有
离子仍能溶解NiS4Pc-Pb(II)沉淀而释放出NiS4Pc,溶液相显示NiS4Pc的特征颜色和吸收。基于这一新发现,本研究建立了高选择性测定
离子的光度分析新方法。相比于常规方法,本法特异性强,稳定性极佳,操作简便快速。此外,本法可实现目视化观测,这对于现场或野外分析尤有价值。
2. 实验部分
2.1. 仪器与试剂
Lambda 25型紫外分光光度计(PerkinElmer,美国);电热恒温水浴锅(上海精密实验设备有限公司);CHN81801型pH计(奥立龙,美国);1 cm石英比色皿;电子分析天平(Sartoriu,德国);L-420离心机(湖南湘仪实验室仪器开发有限公司);XW-80A微型旋涡混合仪(上海沪西分析仪器厂)。
NiS4Pc购自百灵威公司,配制成1.0 × 10−2 mol/L的储备液,贮于4℃备用;包括硝酸铅(上海金山化工厂)、多聚磷酸钠(汕头市西陇化工厂);所用试剂均为分析纯,实验用水为高纯水。荧光计精度为1 nm,pH计精度为0.01个pH单位。
2.2. 实验方法
4 mL离心管中依次加入7.5 mL NiS4Pc (1 × 10−2 mol/L),45 mL Pb(NO3)2 (1 × 10−2 mol/L),加水至3.0 mL,混匀,4000 rpm离心5 min后弃上清,保留沉淀。依次加入水及9.0 mL 0.2 mol/L柠檬酸,混匀后加入多聚磷酸盐溶液或待测样品,使溶液终体积为3.0 mL,溶液于漩涡混合器上混合20 s,4000 rpm离心5 min。取上清液于621 nm处测定其吸光度。无
离子溶液的为空白管,记为A0;含
离子溶液各管为样品管,记为A621,吸光度差ΔA621 = A621 − A0。
3. 结果与讨论
3.1. NiS4Pc的分子结构与吸收光谱
NiS4Pc (图1)是一种在长波区域有强吸收的金属酞菁化合物,其外周的4个苯环上具有四个强极性的磺酸基,故可溶于水,十分适合应用于水相体系。

Figure 1. Molecular structure of NiS4Pc
图1. NiS4Pc的分子结构
NiS4Pc的水溶液在600~700 nm处有一强的特征吸收峰,最大吸收波长位于621 nm处(图2(a)),也是本研究定量分析的测定波长。
3.2. Pb2+对NiS4Pc的沉淀作用
考察了NiS4Pc与常见金属离子Zn2+、Mn2+、Al3+、Ba2+、Cd2+、Pb2+、Ca2+、Cu2+、Ag+、Mg2+、Fe3+、Hg2+、Co2+、La3+、Ce3+的反应,发现其中数种对NiS4Pc具有不同程度的沉淀作用,尤以Pb2+对NiS4Pc的沉淀效果最好。加入Pb2+将NiS4Pc完全沉淀后,扫描上清溶液在相同波长范围内的吸收光谱。可以看出,Pb2+加入后,NiS4Pc几乎完全被沉淀,故上清溶液的吸光度趋于0 (图2(b))。
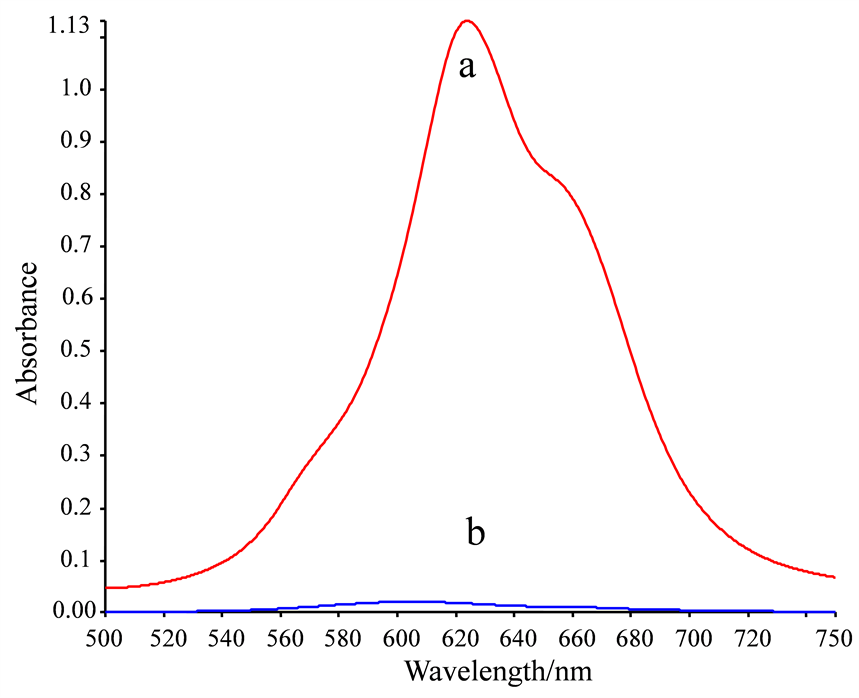 [NiS4Pc] = 2.5 × 10−5 mol/L, [Pb(NO3)2] = 1.5 × 10−4 mol/L.
[NiS4Pc] = 2.5 × 10−5 mol/L, [Pb(NO3)2] = 1.5 × 10−4 mol/L.
Figure 2. Absorption spectra of supernatant of NiS4Pc before (a) and after (b) the addition of Pb2+
图2. NiS4Pc与Pb2+结合前后的吸收光谱
3.3. NiS4Pc对
离子的高选择性线性响应
研究发现,在水相介质中,NiS4Pc-Pb(II)复合物对多种常见的阴离子具有不同程度的响应,即不同酸根离子能不同程度地溶解NiS4Pc-Pb(II)沉淀,使溶液相显现NiS4Pc的特征蓝色。其中,钼酸根、焦磷酸根、三聚磷酸根、偏重亚硫酸根、钨酸根、硅酸根、硼酸根、草酸根、碳酸根和柠檬酸根等酸根离子具有高响应(表1)。

Table 1. The dissolution effect of NiS4Pc-Pb(II) by radical ions
表1. 酸根离子对NiS4Pc-Pb(II)沉淀的溶解作用
[NiS4Pc] = 5.0 × 10−5 mol/L and [Pb(NO3)2] = 8.0 × 10−4 mol/L; the concentrations of all of the acid radical ions were 2 × 10−4 mol/L.
在反应体系中加入低浓度的酸性物质后,发现大多数的阴离子(包括上述高响应阴离子)对NiS4Pc-Pb(II)的溶解作用被明显抑制(表2)。



Table 2. Inhibition effect of acidic substance on the dissolution of NiS4Pc-Pb(II) complex by radical ions
表2. 酸性物质对酸根离子溶解NiS4Pc-Pb(II)沉淀的抑制作用
The concentrations of components in the table S2 were: [NiS4Pc] = 5.0 × 10−5 mol/L and [Pb(NO3)2] = 8.0 × 10−4 mol/L; the concentration of all of the acid radical ions in table S2 was 2 × 10−4 mol/L.
对酸性物质的抑制作用的进一步研究发现,在含有低浓度柠檬酸的介质中,
几乎不受这种抑制作用的影响。也即,在柠檬酸介质中,NiS4Pc-Pb(II)对
表现出特异性响应(图3)。光度测定的结果显示这种响应呈现线性正相关和较高的灵敏度。
 From left to right: a. blank; b. polyphosphate ion; c. acetate ion; d. perchlorate ion; e. chloride ion; f. iodide ion; g. bromide ion; h. nitrate ion; i. fluoride ion; j. dihydrogen phosphate ion; k. nitrite ion; l. periodate ion; m. molybdate ion; n. iodate ion; o. metaphosphoric ion; p. bisulphite ion; q. sulfite ion; r. bicarbonate ion; s. hydrogen phosphate ion; t. carbonate ion; u. bisulfite ion; v. thiosulphate ion; w. tungstate ion; x. sulfide ion; y. silicate ion; z. pyrophosphate ion.
From left to right: a. blank; b. polyphosphate ion; c. acetate ion; d. perchlorate ion; e. chloride ion; f. iodide ion; g. bromide ion; h. nitrate ion; i. fluoride ion; j. dihydrogen phosphate ion; k. nitrite ion; l. periodate ion; m. molybdate ion; n. iodate ion; o. metaphosphoric ion; p. bisulphite ion; q. sulfite ion; r. bicarbonate ion; s. hydrogen phosphate ion; t. carbonate ion; u. bisulfite ion; v. thiosulphate ion; w. tungstate ion; x. sulfide ion; y. silicate ion; z. pyrophosphate ion.
Figure 3. Effect of different kinds of acid radical ions on dissolving NiS4Pc-Pb(II) complex in the presence of low concentration of citric acid
图3. 低浓度柠檬酸存在下酸根离子对NiS4Pc-Pb(II)沉淀复合物的溶解作用
3.4. 反应机理探讨
于NiS4Pc-Pb(II)复合物中再加入
离子,发现NiS4Pc-Pb(II)复合物被迅速溶解。这是由于Pb2+离子与溶液中NiS4Pc发生结合作用形成沉淀,加入
离子后,其与NiS4Pc竞争结合Pb2+离子,导致沉淀溶解,NiS4Pc被释放出来,溶液的光吸收行为得以恢复(图4)。
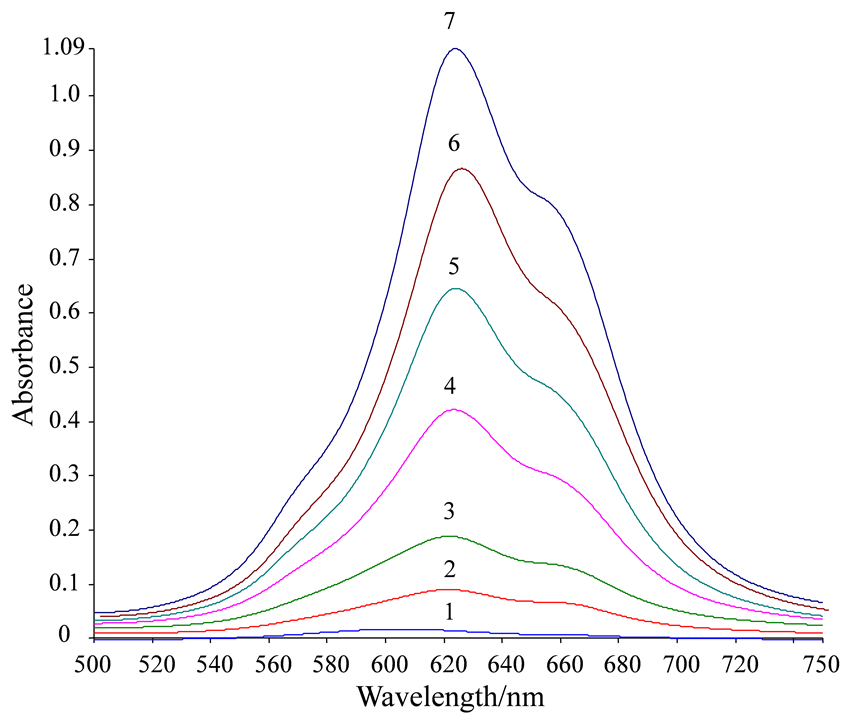 [NiS4Pc] = 2.5 × 10−5 mol/L, [Pb(NO3)2] = 1.5 × 10−4 mol/L, [C6H8O7] = 6.0 × 10−4 mol/L; The concentration of
for curves 1~7 are 0, 3, 15, 30, 45, 60 and 75mg/mL, respectively.
[NiS4Pc] = 2.5 × 10−5 mol/L, [Pb(NO3)2] = 1.5 × 10−4 mol/L, [C6H8O7] = 6.0 × 10−4 mol/L; The concentration of
for curves 1~7 are 0, 3, 15, 30, 45, 60 and 75mg/mL, respectively.
Figure 4. Absorption spectra of the reaction system in the presence of different concentrations of
图4. 不同浓度
存在下体系吸收恢复光谱
柠檬酸能抑制绝大部分酸根离子对NiS4Pc-Pb(II)沉淀复合物的溶解作用,换言之,在柠檬酸存在下,大多数阴离子难以夺取NiS4Pc-Pb(II)沉淀复合物中的铅离子而重新释放NiS4Pc。有报道指出,三聚磷酸根离子可与Pb2+生成可溶性的螯合物,其对Pb2+的螯合作用远强于其他螯合剂或沉淀剂如EDTA,
和
等 [22] 。我们认为,由于
对Pb2+这种强螯合作用,使其在柠檬酸介质中仍能夺取Pb2+而使NiS4Pc游离出来,重新被释放进入溶液体系,体系上清液的吸光度和特征酞菁蓝色得以恢复。基于此现象,建立了NiS4Pc-Pb(II)对
离子的高特异性分析方法。
4. 实验条件的优化
4.1. Pb(NO3)2/NiS4Pc用量比例的优化
考察了Pb(NO3)2、NiS4Pc用量比例对沉淀形成的影响。结果表明,[Pb(NO3)2]/[NiS4Pc]大于2时,NiS4Pc已基本被沉淀。这提示,NiS4Pc与Pb2+可能形成了1:2的复合物。[Pb(NO3)2]/[NiS4Pc]大于4时,NiS4Pc已被沉淀完全。考虑到溶液中过量的Pb(NO3)2可在离心后弃去,故采用[Pb(NO3)2]/[NiS4Pc] > 4进行反应,以保证NiS4Pc被完全沉淀。
4.2. 柠檬酸用量的选择
考察了柠檬酸与NiS4Pc用量比对测定的影响。结果显示,柠檬酸/NiS4Pc比例的变化对三聚磷酸根的响应影响不大,当柠檬酸与NiS4Pc浓度比达到24之后,其对三聚磷酸根以外的酸根离子的抑制效果最佳,故最终选用柠檬酸与NiS4Pc用量比24作为体系中酸性抑制剂的用量。
4.3. 反应时间的选择
实验发现,若NiS4Pc-Pb(II)沉淀复合物在水相中放置时间较长,空白管的上清吸光度A0出现缓慢小幅增加,而扣除空白的样品吸光度差ΔA621则缓慢下降。为保证测定的精确,在反应结束后反应后立即离心,随后移取上清液进行测定。上清液放置3个月后,其颜色和吸光度并无改变,这表明四磺基镍酞菁在水相中的稳定性极佳。
4.4. 反应时间的选择
考察了反应温度对反应体系的影响(图5)。在20℃~80℃范围内,空白管的上清吸光度A0随着温度的升高而增加,这是由于温度升高促进了NiS4Pc-Pb(II)沉淀复合物的离解所致。而扣除空白的样品吸光度ΔA621在30℃~40℃范围内变化甚微,故选择在30℃~40℃下进行反应和测定。
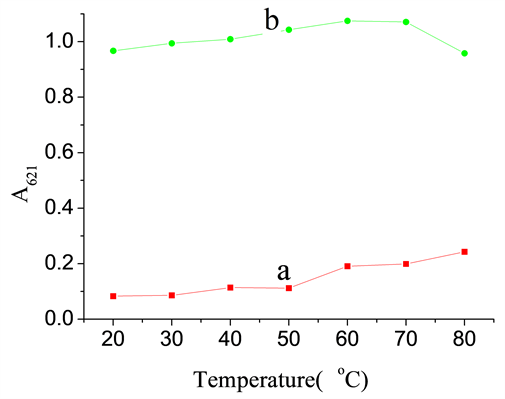 a: A0; b: ΔA621. The concentrations of the components present in the reaction system: [NiS4Pc] = 5.0 × 10−5 mol/L, [Pb(NO3)2] = 3.0 × 10−4 mol/L, [C6H8O7] = 1.2 × 10−3 mol/L, [
] = 1.5 × 10−4 mol/L.
a: A0; b: ΔA621. The concentrations of the components present in the reaction system: [NiS4Pc] = 5.0 × 10−5 mol/L, [Pb(NO3)2] = 3.0 × 10−4 mol/L, [C6H8O7] = 1.2 × 10−3 mol/L, [
] = 1.5 × 10−4 mol/L.
Figure 5. Temperature influence on the absorbance of the supernatant after a reaction
图5. 温度对反应体系吸光度的影响
4.5. 四磺基镍酞菁用量的影响
考察了四磺基镍酞菁用量对方法工作曲线和灵敏度的影响,以
离子的浓度(C, mg/mL)对吸光度差值(ΔA621)绘制标准工作曲线,可以得到不同NiS4Pc浓度下线性回归方程(表3)。结果表明,NiS4Pc浓度为2.5 × 10−5 mol/L时,工作曲线线性区间最大,灵敏度亦较好,故选择NiS4Pc的用量为2.5 × 10−5 mol/L。

Table 3. Optimization for the usage of NiS4Pc
表3. 四磺基镍酞菁用量的优化
4.6. 测定参数
在优化的条件下,测定标准工作曲线。以
离子的浓度(C, mg/mL)对吸光度差值(ΔA621)作图,得到线性回归方程为:ΔA621 = 0.014C − 0.0566。相关系数r = 0.9984,线性区间为3.0~75.0 mg/mL,检测限为2.0 mg/mL。
5. 目视化观测
于4 mL离心管中依次加入7.5 mL NiS4Pc (1 × 10−2 mol/L)溶液,45 mL Pb(NO3)2 (1 × 10−2 mol/L)溶液,加水至3.0 mL,混匀,4000 rpm离心5 min后弃上清;取沉淀,依次加入H2O,9 mL 0.2 mol/L柠檬酸,混匀后依次加入45 mL各酸根离子(1 × 10−2 mol/L)溶液,使溶液终体积为3.0 mL,旋涡震荡器上震荡20 s使溶液充分混匀,4000 rpm离心5 min,取上清液置于一系列10 ml玻璃试管中;空白管为无酸根离子管。加入不同酸根离子的试管中,其颜色差异明显(图3之插图),只有含
离子的试管的颜色表现为水溶性金属酞菁化合物的特征蓝色,其他常见酸根离子与空白管相比无显著差别,表明本法可用于
离子的目视化检测,具有快速、简便、直观的特点。
6. 结论
本研究发现的NiS4Pc-Pb(II)光学探针可实现水溶液中
离子的光度分析,具有高选择性和较好的灵敏度。利用金属酞菁化合物的特征蓝色还可进行目视化观测,对于现场或野外分析具有很强的实用价值。水相中阴离子的高选择性检测、观测一直是分析科学领域的难点和令人瞩目的研究方向,本研究通过制备金属酞菁难溶性化合物光学探针高选择性测定
,在原理上具有普适性,可望借此原理构建更多的光学探针,应用于更多种类阴离子的高选择性检测,对阴离子分析这一研究方向的发展具有重要价值。
致谢
本文得到厦门大学医学院抗研究中心科研条件的大力支持,在此深表谢意。
基金项目
浙江省医药卫生科技计划项目(2018PY036)。
NOTES
*通讯作者。