1. 引言
消化性溃疡疾病(peptic ulcer disease, PUD)是胃部或十二指肠局部黏膜损伤深度超过黏膜基层(muscularis mucosae),导致胃酸持续分泌与刺激,使黏膜损伤后出现症状如上腹部疼痛、心口烧灼、胃酸逆流等的病态 [1]。若黏膜损伤严重,后期亦可能有肠胃道溃疡相关性并发肠胃道出血或穿孔,甚至死亡的现象 [2] [3]。研究指出全球PUD盛行率约为5%~10% [4]。目前除了胃幽门螺旋杆菌(Helicobacter pylori)感染导致消化性溃疡发生之外,服用阿司匹林(Aspirin)或类似的非类固醇类消炎止痛药(nonsteroidal anti-inflammatory drugs, NSAIDs)亦会增加消化性溃疡疾病的发生 [5] [6]。
阿司匹林为常见的NSAIDs之一,具有抗血小板凝集的作用,广泛用于心脑血管疾病的预防,长期使用阿司匹林的患者中,消化性溃疡、消化性出血等胃肠道不良反应发生率明显升高,已引起了临床医师的关注 [7] [8] [9] [10]。阿司匹林具有解热、止痛等效果,其机制主要藉由抑制环氧化酶(cyclooxygenase, COX)的作用,以达到到消炎止痛、抗血栓等效果。而阿司匹林会抑制胃黏膜前列素(prostaglandin, PG)合成,达到解热镇痛的功效,其中前列腺素E1 (PGE1)具有抑制胃酸分泌及增强胃黏膜的抵抗力功用,因此阿司匹林主要藉由PGE1导致胃部溃疡(gastric ulcer, GU) [11] [12] [13]。
益生菌(probiotics)泛指对人体有益的菌,常见为乳酸菌(Lactobacillus spp.)或比菲德氏菌(Bifidobacterium spp.),亦有些像是片球菌(Pediococcus spp.)、芽孢杆菌(Bacillus spp.),以及链球菌 (Streptococcus spp.)等。因能长存于人体肠道中,多半具有耐酸、耐胆盐的特性。益生菌亦多被研究具有肠胃道保护的作用,如减缓肠道发炎、抗菌,或调节肠道菌相影响宿主生理状态等 [14] [15] [16]。这些肠道保护功效会随着菌株(strain)的不同而有不同的强弱效果 [17]。过去实验室自不同的动、植物来源已分离出数株具益生菌特性的细菌,本实验将进一步以复合菌的方式初步筛选具减缓阿司匹林诱发胃溃疡症状的潜力菌株,以利后续益生菌的机能开发与应用。
2. 材料与方法
2.1. 益生菌来源与制备
菌株分离来源与16S rRNA鉴定结果如表1,所有试验菌株皆有取得寄存编号于台湾生物资源保存及研究中心(Bioresource Collection and Research Center, BCRC, Taiwan)。
将各菌株培养于1 L 之MRS培养基(Difco, BD, USA)中,经16小时37℃培养后,以25℃转速5000 rpm (Centrifuge 5804 R, Eppendorf, Germary),离心10分钟取得菌泥,再混入20%脱脂奶粉后冷冻干燥取得活菌菌粉,保存于−20℃备用。各试验复合菌株样品,以各菌粉1:1随机两两组合(表1)。

Table 1. Probiotics sources and grouping
表1. 益生菌来源与复合组合
2.2. 试验动物
8周龄ICR雄性小鼠购自台湾乐斯科生物科技公司(BioLASCO Taiwan Co. Ltd., Taiwan),每只体重约25公克。动物饲养在GLP动物试验室,动物房温度控制23℃ ± 2℃,湿度控制55% ± 5%,光照与黑暗各12小时,饲料及逆渗透水均任由小鼠自由取食。本试验之试验小鼠经台湾农业科技研究院(Agriculture Technology Research Institute, ATRI)之实验动物照护及使用委员会(Institutional Animal Care and Use Committee, IACUC)审查同意,取得IACUC号码109048。
2.3. 试验设计
小鼠经一周适应期后,随机分为11组,每组6只。分别为正常控制组(normal control, N)、负对照组(negative control, NC)、正对照组(positive control, PC)、以及8组复合益生菌组。复合益生菌组以20.4 mg/kg b.w./day连续39天管喂各益生菌,PC组每日以20 mg/kg管喂奥美拉唑(Omeprazole),N组与NC组每日则以饮用水取代之。除N组外,各组于第29天管喂500 mg/kg b.w./day Aspirin连续10天诱发胃溃疡,于第40天以肌肉注射50 mg/mL的舒泰50 (Zoletil 50, Cat. No. 5TK3, Virbac Laboratories, France)牺牲老鼠后,进行各项胃溃疡程度与病理分析。
2.4. 胃溃疡程度分析
取出并剪开小鼠胃部黏膜面拍照记录。利用ImageJ软件将胃部组织的溃疡灶圈出,计算小鼠胃部位溃疡灶面积。胃溃疡面积小于1 mm2给予分数1 (level 1),胃溃疡面积介于1~3 mm2给予分数2 (level 2),胃溃疡面积大于3 mm2给予分数3 (level 3)。胃溃疡程度以胃溃疡指数(Ulcer index, UI) (1)与治愈率(Curative ratio, %) (2)呈现。
(1)
(2)
2.5. 病理分析
利用中性10%福尔马林液固定小鼠胃部组织,将胃腺体部以横切方式修片并放入包埋盒内,经脱水、石蜡浸润及包埋等步骤制成石蜡组织块,以石蜡组织切片机(Leica RM 2145, Nussloch Germany)切成4 m 厚度组织切片,再以Hematoxylin & Eosin (H&E)染色,于光学显微镜下观察各组的胃组织学变化。
以黏膜溃疡、炎症细胞浸润与黏膜上皮再生3项评估指标进行病理分析。病变严重度的判定是参照 Shackelford等人(2002)、Chiu等人(2014)、Hardbower等人(2017),以及Park等人(2018)所发表之文献判定标准为依据 [18] [19] [20] [21]。详细叙述如下:
2.5.1. 黏膜溃疡
表示Aspirin造成胃黏膜上皮细胞发生坏死而导致溃疡病灶的严重度。等级0为正常组织无发生溃疡;等级1为胃黏膜发生表浅层溃疡病灶约为1/3以内;等级2为胃黏膜发生表浅层至中层溃疡约为1/2;等级3为胃黏膜发生表浅层至深层溃疡约为2/3;等级4代表胃黏膜上皮全层溃疡且病灶穿透肌肉层达到3/3。
2.5.2. 炎症细胞浸润
表示Aspirin造成炎症细胞浸润于胃组织病灶区的严重度。等级0代表无炎症细胞浸润;等级1为局部极轻微炎症细胞浸润;等级2为多发局部轻微炎症细胞浸润;等级3为部分区域具连续性中等程度炎症细胞浸润;等级4代表严重炎症细胞浸润。
2.5.3. 黏膜上皮再生
表示Aspirin造成黏膜上皮伤害后之再生现象程度。等级0为无出现黏膜上皮再生;等级1属于极轻微程度;等级2为轻微程度;等级3为中等程度;等级4为严重程度。
2.6. 统计分析
实验结果以平均值(mean) ± 平均标准偏差误差(standard error of the mean, SEM)表示,使用Graphpad Prism 6统计分析软件,进行one-way/two-way analysis of variance统计分析。以*p < 0.05表示具显著差异。
3. 结果
图1为试验期间,每周小鼠的体重变化,结果显示各组间体重变化无显著差异(p > 0.05)。此亦说明测试之复合菌株不影响小鼠的体重变化,以及不会造成小鼠不适。
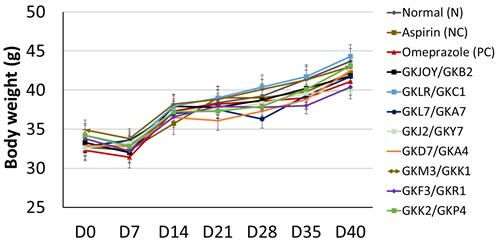
Figure 1. Change of the mice body weight during the experiment
图1. 试验期间小鼠体重变化
3.1. 小鼠胃部溃疡程度
图2(a)为各组小鼠胃部溃疡的面积。结果显示,NC组的溃疡面积为26.01 ± 2.98 mm2显著高于N组,且施予治疗胃溃疡的市售药物奥美拉唑(PC组)后,溃疡面积降为7.12 ± 2.15 mm2,说明此胃溃疡动物模式相当具参考性,可接续讨论本次筛选的复合菌株对于胃保护的效果。
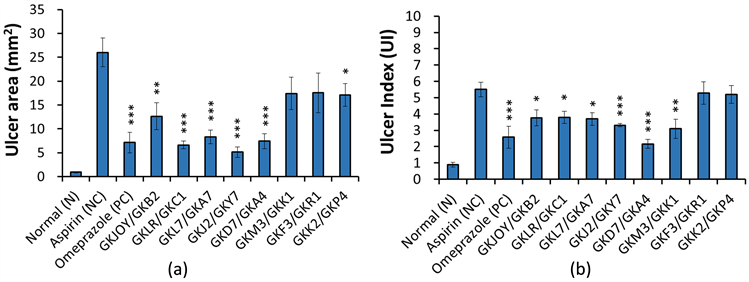
Figure 2. Ulcer area (a) and ulcer index (b) in Aspirin-induced gastric ulcer mice. Data was expressed as mean ± SEM. Significant differences when compared with the Aspirin group (NC) were marked as *p < 0.05, **p < 0.01, and ***p < 0.001
图2. 小鼠胃部溃疡面积(a)与溃疡指数(b)。数据以mean ± SEM呈现,与Aspirin组(NC)比较具显著差异者表示*p < 0.05、**p < 0.01、***p < 0.001
胃溃疡面积结果显示,8个复合益生菌组别在此次的Aspirin诱导胃损伤下皆具有减缓胃部溃疡面积的效果。小鼠经Aspirin造成胃损伤面积较少者为复合菌GKLR/GKC1组、GKL7/GKA7组、GKJ2/GKY7组和GKD7/GKA4组(图2(a))。然而,以溃疡面积加乘计算胃溃疡指数(UI)后,以复合菌GKJ2/GKY7组和GKD7/GKA4组的胃溃疡指数最低,其次为GKM3/GKK1组(图2(b))。将PC组的胃溃疡指数分别与效果最好的两组复合菌组(GKJ2/GKY7、GKD7/GKA4)比较后并没有发现显著差异(p > 0.05)。
以治愈率来看,复合菌GKD7/GKA4组的治愈率最佳,可达60%以上的治愈率,甚至优于PC组的53%,其次依序为GKM3/GKK1、GKJOY/GKB2、以及GKJ2/GKY7,皆有接近40%的治愈率(图3)。

Figure 3. Ulcer cureative ratioin gastric ulcer mice
图3. 小鼠胃部溃疡治愈率
3.2. 小鼠胃部病理状况
小鼠胃部的病理切片如图4所示,可见N组无任何黏膜溃疡、炎症、或黏膜再生的情况;然而,NC组有的老鼠胃部呈现严重的黏膜溃疡、发炎浸润,以及严重的细胞病变坏死等情况。PC组则虽有部分溃疡等情况,但普遍比NC组的溃疡程度还来得低。而复合菌组别皆观察到较少的发炎或溃疡现象。
将黏膜溃疡、发炎细胞浸润及黏膜上皮再生之病理评分加总后呈现于图5。与Asprin组(NC)相比,测试的复合菌株中,以GKLR/GKC1最为显著减少胃部组织病变上的分数(p < 0.05) (图5)。虽然GKD7/GKA4组没有与NC组产生统计上的显著差异,但仍然也有降低病变评分的趋势。
4. 讨论
消化系统溃疡的成因主要是黏膜的保护因子与攻击(破坏)因子失去平衡所造成的。黏膜的保护因子包含黏液、重碳酸盐分泌、细胞再生、血管新生、黏膜血流、前列腺素合成等;而黏膜的攻击因子则包含胃酸、胃蛋白、胆酸、缺氧、压力、幽门杆菌、消炎止痛药、烟酒、咖啡等刺激物。其中,NSAIDs药物的作用在于减少胃黏膜之厌水性(hydrophobicity),使胃酸与胃蛋白酶可轻易穿透胃黏膜造成伤害 [11]。研究指出前列腺素(prostaglandins)对胃黏膜的维持与修复扮演重要的角色,而NSAIDs药物的副作用归咎于对环氧化酶(COX)的抑制作用,间接抑制前列腺素之产生而达到止痛消炎作用,导致胃黏膜前列腺素的合成造成黏膜伤害 [2] [22]。
肠胃道透过黏膜屏障包含物理屏障、化学屏障和生物屏障等,来达到保护的作用。高等人(2012)研究指出,益生菌在保护阿司匹林致大鼠胃黏膜损伤的作用机制其一为通过丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)中的p38信号通路上调胃黏膜上皮黏膜紧密连接蛋白Occludin的表达,进而增强了大鼠胃黏膜的物理屏障功能 [23]。另外,肠道菌群在NSAIDs胃病的发生亦起重要作用,大量的NSAIDs应用会改变肠道菌相,像是变形杆菌和厚壁菌等的比例增加,引起肠胃不适;因此施予益生菌,像是乳酸杆菌或双歧杆菌具一定的菌相平衡作用,提高受损黏膜的复原效果,藉由增加生物屏障达降低肠胃的不适 [24]。
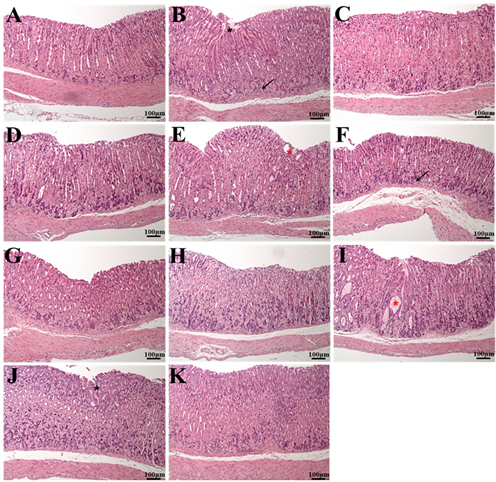 (A) 正常对照组(N);(B) 负对照组(NC):(C) 正对照组(PC);(D) GKJOY/GKB2组;(E) GKLR/GKC1组;(F) GKL7/GKA7组;(G) GKJ2/GKY7组;(H) GKD7/GKA4组;(I) GKM3/GKK1组;(J) GKF3/GKR1组;(K) GKK2/GKP4组。放大倍率100倍。(黑色星号:黏膜上皮再生区域;黑色箭头:炎症细胞浸润;红色星号:细胞变性坏死区域)。
(A) 正常对照组(N);(B) 负对照组(NC):(C) 正对照组(PC);(D) GKJOY/GKB2组;(E) GKLR/GKC1组;(F) GKL7/GKA7组;(G) GKJ2/GKY7组;(H) GKD7/GKA4组;(I) GKM3/GKK1组;(J) GKF3/GKR1组;(K) GKK2/GKP4组。放大倍率100倍。(黑色星号:黏膜上皮再生区域;黑色箭头:炎症细胞浸润;红色星号:细胞变性坏死区域)。
Figure 4. H&E staining in mice stomach
图4. 小鼠胃组织H&E染色
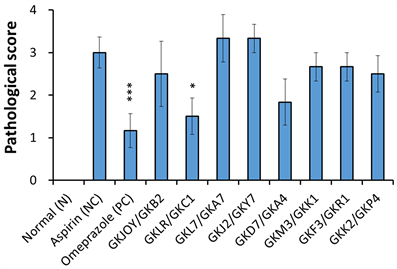
Figure 5. Pathological scoring in mice stomach. Data was expressed as mean ± SEM. Significant differences when compared with the Aspirin group (NC) were marked as *p < 0.05, **p < 0.01, and ***p < 0.001
图5. 小鼠胃部病理评分。数据以mean ± SEM呈现,与Aspirin组(NC)比较具显著差异者表示*p < 0.05、**p < 0.01、***p < 0.001
在本次的筛选中,可见不同菌株在影响胃保护作用上的差异性,像是减少溃伤面积(图2)、病变程度(图4)。后续会针对菌株Lactobacillus plantarum GKD7和Pediococcusacidilactici GKA4再做一次胃保护的试验,分别出最有胃保护效果的菌株,以及其相关的机制探讨。
5. 结论
本试验利用复合菌株提高筛选基数,作为初步筛选具胃部保护潜力的益生菌。实验分别以胃部溃疡程度及病理切片讨论复合菌摄入4周后于Asprin诱导下的小鼠之胃部保护效果。结果复合菌GKJ2/GKY7和GKD7/GKA4显著减缓胃溃疡指数;以治愈率而言,又以GKD7/GKA4组改善效果最佳。病理评分上,则以复合菌GKLR/GKC1改善最为显著,其次为复合菌GKD7/GKA4。综观上述实验结果,本筛选以复合菌GKD7/GKA4组为佳。后续将分别以Lactobacillus plantarum GKD7和Pediococcus acidilactici GKA4进行关于胃部保护的深入研究。
NOTES
*通讯作者。